Glycolysis, Gluconeogenesis, Fate of pyruvate, Alcohol metabolism, PPP, Glycogen
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
Glycolysis oxidizes ________ into __________ and generates ______ through ________-______ _______________
Glycolysis oxidizes glucose into pyruvate and generates ATP through substrate-level phosphorylation
Glucose Transporters
what do they do?
What are the types and what tissue does it belong to?
Which transporter is sensitive/dependent on insulin? (need insulin to work)
Transport glucose across the membrane for glycolysis
SGLT→ Renal tubules
GLUT1→ Pancreatic beta cells and hepatocytes
GLUT2→ Pancreatic cells, hepatocytes and kidney
GLUT3→ CNS
GLUT4→ skeletal muscle, cardiac muscle and adipose tissue
Only GLUT4 (sket/card muscle + adipose) is sensitive to insulin
Is glycolysis anaerobic or aerobic?
Does it occur in the fed or fasting state?
Where does it occur
Anaerobic
Fed
Cytosol
How is glycoslysis regulated?
Allosteric regulation and hormonal regulation
Aside from ATP, what else does glycolysis generate?
Precursors for fatty acid biosynthesis, amino acids and nucleotides
What are the phases of glycolysis?
What is the net yield?
Preparative Phase: 2 ATP’s are invested
ATP-Generative Phase: Generates 4 ATP and 2 NADHs
Net yield: (4-2) ATP, 2 NADH, 2 Pyruvate
What steps of glycolysis are heavily regulated?
List the enzyme and how it is regulated allosterically vs hormonally
1, 3, 10
Hexokinase: +Glucose, - G6P
Glucokinase: +Glucose, - F6P
PFK: + ADP, AMP, F2,6-bsp; - ATP, citrate
PEP kinase: +f1,6-bsp; - ATP, Acetyl CoA, LFCA, Alanine
Hexokinase vs Glucokinase
Km
Vmax
Location
Hexokinase has a lower Km and lower Vmax
Thus it binds glucose better at low concentration while Glucokinase binds better and more at higher concentrations
The liver only takes up glucose when there is a surplus
Hexokinase is everywhere and Glucokinase is in the liver only
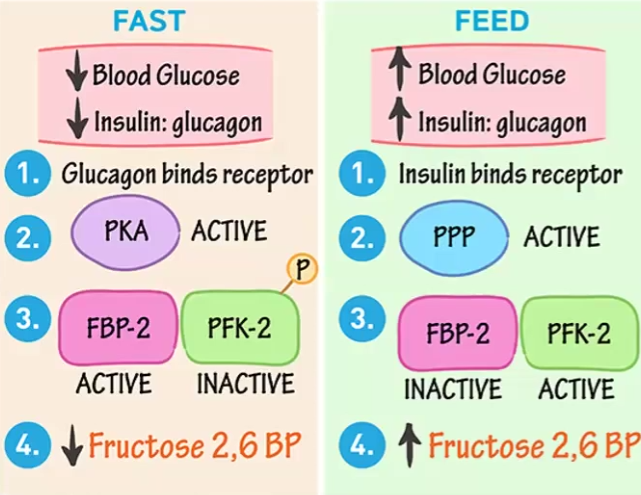
Fructose 2,6- bisphosphate feeding vs fasting
What enzymes converts Fructose 6 phosphate into Fructose 1,6-bisphosphate
PFK2
Fasting
Insulin increases and activates PKA, which phosphorylates PFK2 and renders it inactive, thus F2,6-bsp activity decreases
Feeding
Glucagon is active and activates PPP, which activates PFK2 and increases F2,6-bsp activity
Is there an alternative Fructose metabolism?
What enzymes can be deficient?
yes
fructokinase → benign deficiency as it can be easily replaced by the alternative pathway
Aldolase B → severe effects because there is only this aldolase to perform the reaction
Nonclassical vs. Classical Galacticemia
Nonclassical → galactokinase deficiency causes galactose accumulation which in the eyes gets transformed into galacticitol which causes cataracts
Classical→ galactose 1-phosphate accumulates and causes the same but more serious and early symptoms
What are the fates of pyruvate
What is the main purpose?
aerobic → TCA → E transport chain
anaerobic → Lactate
Regenerating electron carriers (NAD+ from NADH) and creating ATP
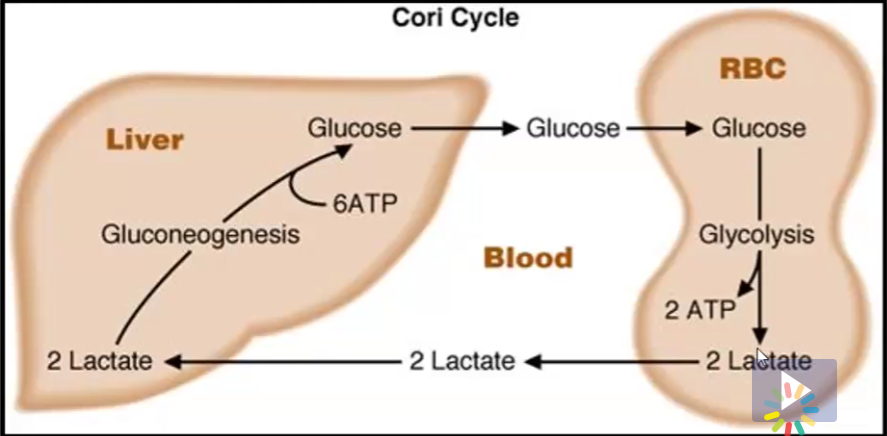
Cori Cycle
what other tissue can use lactate, what does it turn it into?
Tissues such as RBC’s create lactate because they lack mitochondria
They however cannot use this lactate
In the Cori Cycle, this lactate is shuttled to the liver, where it can be used for gluconeogenesis and turned back into glucose so the lactate generating tissues can use it again
the heart, turns lactate into pyruvate
Gluconeogenesis
What is the starting substrate?
What are the precursors to the starting substrate?
Where does it happen?
What state does it happen in?
How many steps are different from glycolysis?, which ones?
Pyruvate
Lactate, Amino acids such as alanine
In the liver
Fasting state
3 steps are different from glycolysis
Pyruvate → Phosphoenol pyruvate; Fructose 1,6-bisphosphate → Fructose 6 phosphate; Glucose 6 phosphate → Glucose
What are the gluconeogenesis enzymes and their inhibitors
Pyruvate carboxylase → (+) Acetyl coA (-) ADP
Phosphoenol pyruvate carboxylase kinase (PECK) → (-) ADP
Fructose 1-6 bisphosphatase → (-) Citrate and (-) AMP
Glucose 6 phosphatase → (+) Glucose 6 phosphate
Alcohol and Hypoglycemia
What reaction causes it
What is the main presentation of alcoholic patients, why?
Alcohol is converted to acetaldehyde and produces NADH, which tricks the body into thinking they have enough energy and thus stopping TCA and gluconeogenesis
Although there is plenty of NADH, there is no metabolic fuel → glucose → thus the alcoholic patient becomes hypoglycemic (not enough sugar)
Because gluconeogenesis is inhibited, lactic acid builds up and the main presentation of alcoholic patients is lactic acidosis
What are the other two names for PPP?
What does the PPP bypass?
What is the starting substrate?
Where does the PPP happen in the cell?
Hexose Monophosphate Shunt and HMP Shunt
It bypasses the first stage of glycolysis
The starting substrate is glucose 6 phosphate (G6P)
In the cytosol
What does the PPP generate?
What is it used for?
Generates NADHP, glycolysis intermediates and nucleic acid precursors
NADHP is used for nucleotide synthesis, as a glutathione reductase (peroxidase) cofactor, for superoxide mutase reactions, for lysosome phagocytic activities, for nitric oxide synthesis, for fatty acid synthesis (steroid hormones from cholesterol), and for liver detoxification
Explain glucose 6 phosphate dehydrogenase deficiency
What other chemicals can help against oxidative stress?
If this enzyme is deficient, the oxidative phase of PPP cannot happen thus there is insufficient NADPH in the cell, which in turn results in glutathione reductase activity decreasing which means there wont be enough glutathione to prevent peroxide/oxidization damage from happening to RBCs
This causes hemolytic anemia, which means RBC’s are lysed due to oxidization
Ascorbate (Vitamin C), Vitamin E and Beta carotene
Regulation of PPP
Determine the direction of the pathway depending on the following cell needs
NADPH only
NADPH and ribose 5 phosphate
Ribose 5 phosphate only
NADPH and pyruvate
NADPH only → oxidative to produce NADPH and non-oxidative to go back to oxidative to make more NADPH
NADPH and ribose 5 phosphate→ both as NADPH requires both and ribose5p requires non-oxidative
Ribose 5 phosphate only → only non-oxidative because we can start from a glycolysis intermediate since the pathway is reversible
NADPH and pyruvate → both because NADPH requires both and pyruvate requires non-oxidative as it creates glycolysis intermediates
Glycogen
What type of glycogen does not get degraded during fasting?
How are glucosyl units joined?
How are they branched?
What does branching allow?
What are the two types of end, what are their purposes?
Skeletal muscle glycogen
Joined by alpha 1,4 glycosidic bonds
Branched every 8-10 residues by alpha 1,6 glycosidic bonds
Branching allows fast degradation at multiple points
Reducing end has the glycogenin which creates the primer for synthesis, Non-reducing end is where UDP glucose is added or G1P is removed
What are the enzymes of glycogenesis?
What about glycogenolysis?
The enzymes of glycogenesis are glycogenin for the primer, glycogen synthase and branching enzyme (transferase)
The enzymes for glycogenolysis are glycogen phosphorylase and branching enzyme (transferase activity and alpha 1,6 glucosidase activity)
Glycogen Storage Diseases
Type O → glycogen synthase deficiency
Type I → glucose 6 phosphatase deficiency → Von Gierke Disease
Type II → lysosomal alpha amylase deficiency → Pompe Disease
Type III →amylase 1,6 glucosidase deficiency (debranching enzyme)
Type IV → amylase 4,6 glucosidase deficiency (branching enzyme → Andersen’s Disease
Type V → muscle phopshorylase deficiency → McArdle Disease
Type VI → liver phosphorylase → HERs disease
Type VII → PFK1 deficiency → Tarui’s Syndrome
Type XI → GLUT 2 deficiency → Fontani' and Bickel Syndrome
Hormonal Regulation of Glycogen
Glucagon + cAMP + PKA + which inactivates glycogen synthase (by phosphorylation) and activates glycogen phosphorylase (by dephosphorylation)
Insulin + PPP + which inactivates glycogen phosphorylase (by phosphorylation) and activates glycogen synthase (by dephosphorylation)
Allosteric regulation of glycogen
Glycogen synthase → +Glucose 6 phosphate
Glycogen synthase → +AMP or Ca+ and - by ATP, G6P or glucose