homologus series : alkanes and alkenes
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
What are alkanes and alkenes ?
They are hydrocarbons that are 2 examples of homologous series of compounds
What happens in a homologous series ?
the hydrocarbons share the same general formula
each molecule differs by CH(lil2) from it’s neighbouring (successive) molecule
have similar chemical properties
show gradual variation (trends) in physical properties
Give the general formula of an alkane ? Think chemically and mathematically
chemically - CnH2n+2
mathematically - CxHy=2x+2
Explain what an alkane is ?
Alkanes are molecules ending in “ane” - they only have single bonds which means they are saturated.
What does it mean if a molecule is saturated ?
it means they are full and there are no double bonds present
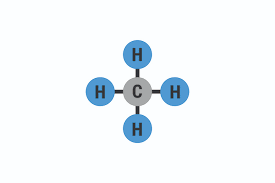
What is the formula, structure, formula Mass and boiling point for METHANE ?
FORMULA: CH(lil4)
STRUCTURE: look at image
FORMULA MASS: 16
BOILING POINT: -164
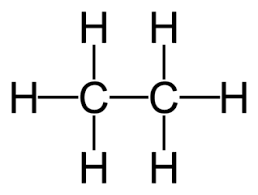
What is the formula, structure, formula Mass and boiling point for ETHANE ?
FORMULA: C(lil2)H(lil6)
STRUCTURE: look at image
FORMULA MASS: 30
BOILING POINT: -88
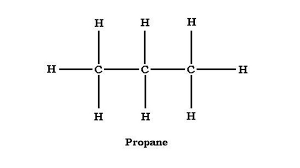
What is the formula, structure, formula Mass and boiling point for PROPANE ?
FORMULA: C(lil3)H(lil8)
STRUCTURE: look at image
FORMULA MASS: 44
BOILING POINT: -42
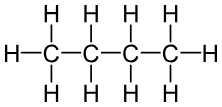
What is the formula, structure, formula Mass and boiling point for BUTANE ?
FORMULA: C(lil4)H(lil10)
STRUCTURE: look at image
FORMILA MASS: 58
BOILING POINT: 0
Explain what an alkene is ?
They are molecules ending in “ene” and they have 1 double bond between carbons and are unsaturated
Give the general formula for an alkene ? Think chemically and mathematically
chemically - CnH2n
mathematically - CxHy=2x
What is cracking ?
it is where we take longer less useful chains and we split them into shorter more useful chains and alkenes which are in higher demand
What do we use short chain products for ?
they are generally used in fuels and the alkenes (unsaturated molecules) used to make polymers
Why is cracking necessary ?
General the demand for certain fractions outstrips the supply so this is why cracking is necessary to convert surplus unwanted fractions into more useful ones
This is mostly larger, heavier fractions that are cracked into smaller lighter fractions