Year 10 GCSE Chemistry - Introducing Chemical Reactions
1/72
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
73 Terms
Avogadro constant
Number of entities in one mole, equal to the number of atoms in 12.0 g of ¹²C atoms, 6.02 * 10 ^ 23 (3 s.f.)
Balanced equation
Model for a reaction showing formulae using symbols, where the numbers of each atom are the same on both sides of the equation. how atoms are rearranged and the relative amounts of each substance involved
Closed system
Apparatus in which substances cannot enter or leave the reaction mixture during it
Compound ion
Ion formed when a group of atoms loses or gains electrons
Half equation
Type of chemical equation that models the change that happens to one reactant in a reaction
Ionic equation
Type of chemical equation that models how oppositely charged ions form an ionic compound
Law of conservation of mass
Principle stating that the total mass remains constant during a chemical reaction, as atoms are not created or destroyed during a chemical reaction, just joined in a different way
Limiting reactant
Present in an amount less than needed to react completely with the other reactant in a chemical reaction, stopping it
Molar mass
Mass in grams of one mole of a substance
Mole
Unit for amount of a substance containing the same number of particles as there are atoms in 12.0 g of ¹²C, 6.02 * 10 ^ 23
Molecular formula
Description of a compound or an element using symbols for atoms and numbers to show the actual number of each element in a molecule
Non-enclosed system
Apparatus in which substances can enter or leave the reaction mixture during it
Precipitate
Insoluble product in the solid state, formed during a reaction involving solutions
Precipitation
Type of reaction in which a precipitate forms
Spectator ion
Charged particle present in a reaction mixture but does not take part in it
State symbol
Letters used to represent the physical state of a substance
aq, example of four state symbols
Means substance is dissolved in water
Stoichiometry
Describes the relative amount of each substance involved in a chemical reaction, the mole ratio; how many moles are reacting with each other
Word equation
Model of a reaction that describes reactants and products using their chemical names
Dmitri Mendeleev
Russian chemist who spent a significant part of his working life, in the second half of the nineteenth century, trying to arrange the chemical elements into some order
What Mendeleev's arrangement was not the first attempt by chemists to do
Organise the elements into a table
What Mendeleev's periodic table was, and what it lead to
The most successful, our modern Periodic Table
Mendeleev had been considering his own and other scientists' -
Evidence about the elements for several years before his first table
Four things Mendeleev's periodic table achieved
Put elements in order of atomic mass, arranged them in columns with similar chemical properties, left gaps for elements yet to be discovered, predicting their properties and switched tellurium and iodine round to make their properties match other elements in the group
Why the modern periodic table has elements arranged in order of atomic number, not atomic mass
Mendeleev did not know about protons, explaining the leaving of gaps and swapping Te and I
What missing elements discovered fit, and how we know
Gaps he left, having his predicted properties
Why noble gases were not discovered in Mendeleev's time
Very unreactive
What Mendeleev changed about his periodic table by 1871
It currently showed groups as rows, so he rotated he rotated the table so groups were in columns
When three of Mendeleev's predicted elements were discovered
Between 1875 and 1886
Who an when discovered that an atom's atomic number was actually the number of protons in its nucleus
Henry Moseley in 1913
Henry Moseley
English physicist
Two things Moseley's work explained or showed there were in the 1913 Periodic Table
Seven gaps to fill and explained why Mendeleev had been right to swap tellurium and iodine
Johann Döbereiner, 1828 - timeline of the Periodic Table
Developed 'triads', groups of three elements with similar properties
Elements forming triad 'number one'
Lithium, sodium and potassium
Elements forming triad 'number two'
Calcium, strontium and barium
Elements forming triad 'number three'
Chlorine, bromine and iodine
John Newlands, 1865 - timeline of the Periodic Table
The known elements, more than sixty, were arranged in order of atomic weights and observed similarities between the first and ninth elements, the second and tenth elements and so on, proposing the 'Law of Octaves'
Three elements with gaps left for, unknown at the time but their properties predicted, Dimitri Mendeleev, 1869 - timeline of the Periodic Table
Gallium, scandium and germanium
William Ramsay, 1894 - timeline of the Periodic Table
Discovered Noble Gases
When Henry Moseley, 1913, was killed - timeline of the Periodic Table
During the first World War
What the result of Moseley being killed in the first World War was
No leading British scientist is now allowed into a situation where there life may be lost during war time
When - ide is used when naming compounds
If it contains just two elements
When - ate is used when naming compounds
If it contains three or more elements, one of which is oxygen
Number of letters in a chemical symbol
One or two
Why the formulae for metal elements are always written as a single symbol
There are too many atoms in a giant metallic lattice to write the numbers involved
What non-metal elements in group zero exist as
Individual atoms, attracted to each other by weak intermolecular forces
What non-metal elements in group seven exist as
Diatomic molecules, attracted to each other by weak intermolecular forces
Diatomic molecule
Contains two atoms covalently bonded together
Three diatomic molecules not in group seven
Hydrogen, nitrogen and oxygen
What other non-metal elements, such as carbon and silicon exist as
Giant covalent lattices
What other non-metal elements, such as sulphur, exist as
Large molecules
What these other non-metal elements [those forming giant covalent lattices and large molecules] are notated using
The chemical symbol for these elements
Exception to using chemical symbol for these other [those forming giant covalent lattices and large molecules] non-metal elements
Phosphorus, P₄
What the molecular formula for a simple covalent compound shows
Symbols for each of the elements it contains and number of atoms of each elements in one of its molecules
Ensure I know formulae of common ions, find on page titled "Formulae of Common Ions to Learn"
Completed
Hallogens
Group-seven elements
Transition elements can be -
Shaded
Charges of transition-metal ions
Can vary but are given by roman numerals in brackets e.g. Fe(II) = Fe²⁺ ions
What an ionic compound contains
Positive and negative ions
Why knowing a substance is an ionic compound allows you to find its chemical formula
It has no overall charge - this fact can be used
Example of chemical reaction where mass will decrease, if carried out in a non-enclosed system, and why
Calcium carbonate chips in dilute hydrochloric acid, where bubbles of carbon dioxide would then escape
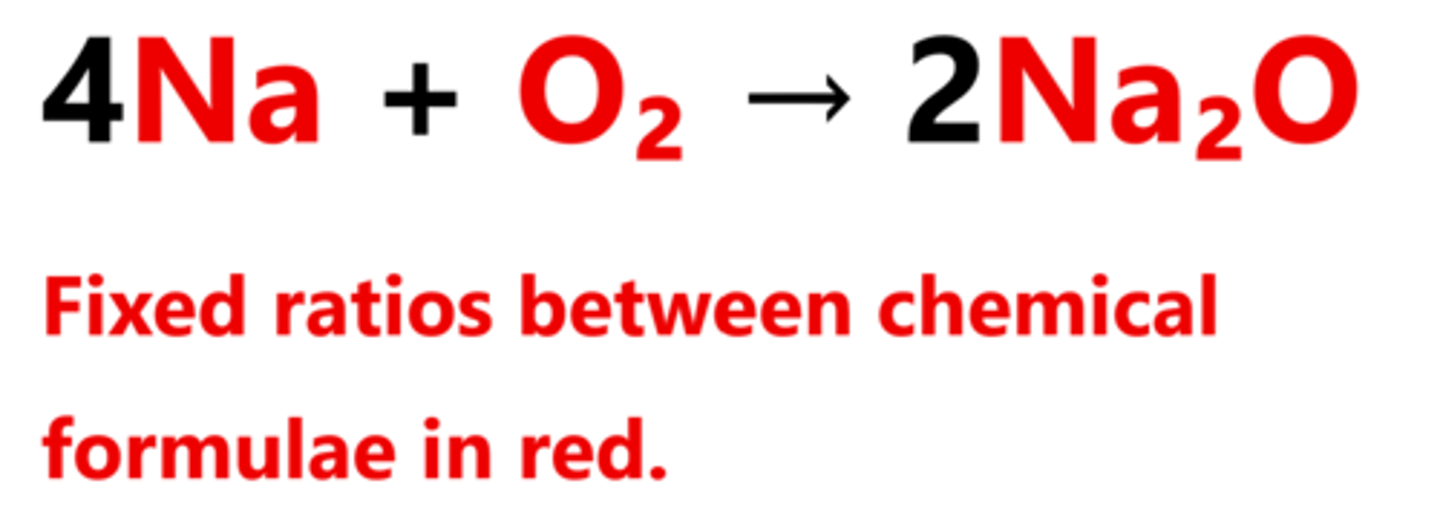
What there are between the substances in a chemical reaction, ignoring coefficients. Example is shown in image attached
Fixed ratios between them
State that sodium metal reacts with water in
Liquid
What sodium metal reacting with water produces
Sodium hydroxide dissolved in water and hydrogen in the gas state
Example of balanced chemical equation using state symbols, that expresses the word equation sodium + water → sodium hydroxide + hydrogen
2Na(s) + 2H₂₂O(l) → 2NaOH(aq) + H₂(g)
Mass of one mole of a substance =
Molar mass, measured in g mol ^ - 1, or its relative atomic mass or relative formula mass
moles =
mass / Mᵣ
Two things moles can be used to calculate
Mass of product made or reactant needed in a chemical reaction
What you do not include when calculating reacting mass equations, when finding Mᵣ values
Coefficients
Reactant in excess
One that is not used up, present in an amount greater than that needed to react with the other reactant
Type of reactant used to calculate reacting masses
Limiting
What Mᵣ is the sum of multiple, when calculating
Relative atomic masses
Mᵣ
Relative atomic mass of one unit of a compound