chemistry - key concepts in chemistry: the periodic table (1.13 - 1.20)
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
1.13 how did Mendeleev arrange elements in periodic table?
in order of increasing RAMs
sometimes swapped positions of elements - better suited chemical properties of elements & their compounds
assumed more elements would be discovered - left gaps
vertical columns - increasing RAM
rows - similar chemical properties
1.14 how did Mendeleev predict existence & properties of some undiscovered elements?
used gaps in table - based on properties of nearby elements
1.15 why was Mendeleev’s order not always correct?
thought he arranged elements in order of increasing RAM
not always true due to relative abundance of isotopes of some pairs of elements - affects RAM
1.16 atomic number - position in periodic table & protons
elements (in row) in order of increasing atomic number
atomic number = number of protons
1.17 periodic table arrangement
elements in order of increasing atomic number in rows (periods)
elements with similar properties in same columns (groups)
1.18 position of metals vs non-metals in periodic table & why
metals - left & middle
less electrons in outer shells
lose electrons easily (form cations)
non-metals - right
more electrons in outer shells
gain electrons easily (form anions)
1.19 predict electronic configurations
e.g. chlorine:
number of electrons = 17
2.8.7
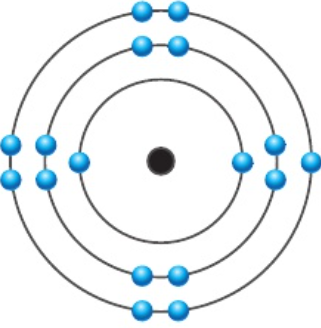
1.20 how is electronic configuration related to position in periodic table?
number of occupied shells = period number
number of valence electrons = group number (group 0 = full outer shells)