moles, empirical formula, molecular formula, chemical reactions (practice balancing)
1/8
Earn XP
Description and Tags
Flashcards for Chemistry Test Review: Moles, Empirical Formula, Molecular Formula, and Chemical Reactions.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
9 Terms
Calculating Mole Given grams of element = ? mole of element
The amount of a substance containing the same number of particles as there are atoms
Calculate molar mass of element
1 mol element = molar mass of element
given grams * 1 mol element/molar mass of element = mol of grams
Particles
Atoms = single neutral particles (ex na)
Molecules = neutral particles of two or more atoms onded together (ex h2o)
Ions = positively/negatively charged particle (ex na+)
Formula units = the simplest ratio of ions in an ionic compound. (ex kcl)
Mass Percent Composition
The percent by mass of each element in a compound.
Molecular Formula
The actual number of atoms of each element in a molecule.
Main chemical reactions
Synthesis : A+B → AB
Decomposition: AB → A+B
Single replacement: AB + C → AC + B
Double replacement: : AB + CD → AD + CB
Combustion: A + O₂ → CO₂ + H₂O
empirical formula process
is the method used to determine the simplest whole number ratio of elements in a compound. This involves converting the mass of each element into moles, dividing by the smallest number of moles, and then using these ratios to write the empirical formula.
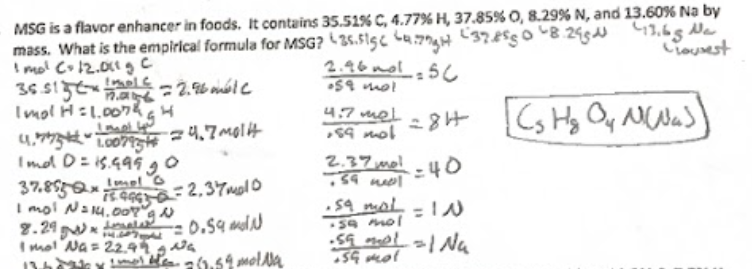
molecular formula process
is the method used to determine the actual number of atoms of each element in a compound. It may involve multiplying the empirical formula by a whole number to reflect the molecular structure.
given grams/Molar mass of compound=subscript for each element to find the ratio of elements in the molecular formula.
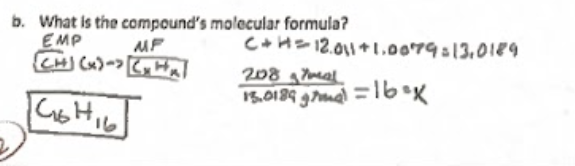
mass percent composition process
is the method of calculating the percentage by mass of each element in a compound. This is done by dividing the mass of each element by the total molar mass of the compound and multiplying by 100.
when asked for grams of each element, use the mass percent to find individual contributions. Given gram * mass percent composition = g of element
