States of Matter Weeks 7-12 ILOs and Concepts
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
57 Terms
Describe the variables of state.
Variables of state are the variables needed to specify the state of a system. They include:
amount of a substance it contains, n
volume it occupies, V
pressure, p
temperature, T
Explain how the partial pressures or gases in a mixture relate to total pressure.
Total pressure = sum of partial pressures of each component
P = pa + pb + pc + …
Rationalise how pressure changes as a result of changing the number or speed of molecules.
Pressure increases if number or speed of molecules increases as more collisions with the container walls and each other occur.
Work
Done in order to achieve motion against an opposing force
Kinetic energy
Energy as a result of motion
Potential energy
Energy as a result of its position
Total energy
Sum of kinetic energy and potential energy
Coulomb potential energy
Energy between 2 charges separated by a distance r. Varies as 1/r
Boltzmann distribution
Formula for calculating the relative positions of states of various energies

Boyle’s law
p inversely proportional to V
Charles’ Law
V proportional to T
p proportional to T
Avogadro’s principle
V proportional to n
Physical state
Its form (soild, liquid or gas) under the current conditions of pressure, volume and temperature
Equation of state
An equation that interrelates the variables that define the state of a substance
Perfect gas
Gas that obeys the perfect gas law under all conditions
molecules of a gas behave identically
molecules are hard, perfectly elastic spheres
volume of molecules negligible compared to the volume of the container
no intermolecular forces between the molecules
molecules move in continuous random motion
Explain the features of the Maxwell-Boltzmann distribution of speeds
y axis: percentage/proportion of particles, x axis = energy
highest point is where most of the particles are found
higher temperatures - curve moves right and down
Describe how molecules behave in perfect and real gases
In perfect gases, molecules behave with no intermolecular forces whereas real gases have intermolecular forces and the volume of real gases is no longer negligible as it is in perfect gases. Real gases deviate from perfect gases at high pressures and low temperatures.
Interpret experimental isotherms and use them to identify the values of critical constants
Experimental isotherms plot pressure against molar volume at different temperatures. They usually show that at small molar volumes gases deviate more from the perfect gas isotherm
Kinetic model of a gas
Considers only the contribution to the energy from the kinetic energies of the model and accounts for the equation of state of a perfect gas
Maxwell-Boltzmann distribution of speeds
Gives the fraction of molecules that have speeds in a specified range
Collision frequency
Average number of collisions made by a molecule in a period of time divided by the length of that period
Mean free path
Average distance a molecule travels between collisions
Compression factor
Helps summarise the extent of deviations from perfect behaviour
compression factor = measured volume (molar volume)/calculated volume of a perfect gas

Virial equation
Empirical extension of the perfect gas equation that summarises the behaviour of real gases over a range of conditions

Critical temperature
Gas can be liquefied by pressure alone if temperature is at or below the critical temperature
Van de Waals equation
Model equation of state for a real gas
Interpret and describe the features of phase diagrams
T3 is the triple point where solid, liquid and gas all exist in equilibrium and is at a specific temperature and pressure for each substances. The triple point is also the lowest pressure at which the liquid phase of a substance can exist.
Tc is the critical point where the interface/boundary between liquid and vapour disappears and a single, uniform phase called a supercritical fluid is left.
Phase transition
Spontaneous conversion of one phase into another
Phase
Form of matter that is uniform throughout in chemical composition and physical state
Phase diagram
Indicates the values of pressure and temperature at which a particular phase is most stable or is in equilibrium with other phases
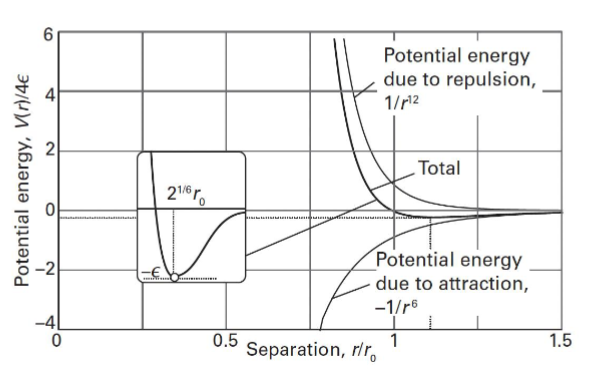
Lennard-Jones potential energy
Model of total intermolecular potential energy, including repulsion
Equation:
V=4(eplisom)(r012/r - r06/r) where r0 is the separation when V=o and r is separation
The minimum on the Lennard-Jones potential energy diagram is the most stable, lowest energy state for the molecule and where attractive and repulsive forces are equal.
Atoms or ions can be modelled as spheres in solids. Spheres cannot fill all space: gaps always remain and so close packing gives an arrangement that minimises empty space.
Coordination number is the number of spheres immediately surrounding another sphere. Coordination number can give the type of structure and how efficiently spheres are arranged.
Molecular solid
Solid consisting of discrete molecules held together by van de Waals forces, possibly hydrogen bond
Covalent solid
Solid that forms an extended network through covalent bonds and the whole crystal system behaves like a giant molecule
Metal
Atomic orbitals overlap to form a band which is a set of molecular orbitals that are closely spaced with infinitesimally small gaps between orbitals and cover a finite range of energy. Electrons occupy the orbitals within the band.
Close packed layer
Layer of spheres arranged so there is a maximum utilization of space
Hexagonally close packed structure (hcp)
Structure is ABABAB… with third layer directly on top of first layer by placing second layer on up gaps of first layer and third layer on down gaps of second layer
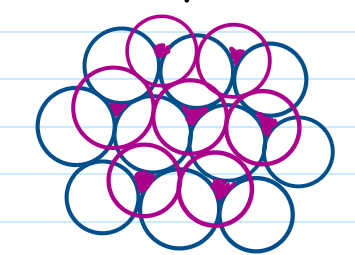
Cubic close-packed structure (ccp)
Structure is ABCABC… Third layer is not directly above the first layer but instead above the gaps in the first layer. Again the second layer is on the up gaps of the first layer but the third layer is also on up gaps of second layer.
Capillary action
Tendency of liquids to rise up and down narrow tubes. If cohesion is greater than adhesion, moves down. If adhesion is greater than cohesion, rises up narrow tubes.
Nucleation
Provides surfaces to which molecules can attach and induce condensation
Colligative properties
Depends only on the number of solute particles present, not their identity.
Osmotic pressure
Minimum pressure that must be applied to a solution to stop the flow of solvent molecules (water) through a partially permeable membrane
Temperature-composition diagram
Phase diagram where the boundaries show the composition of the phases that are in equilibrium at various temperatures. Contain dew point line (top line, where last drop of liquid is - where condensation starts/end) and bubble point line (bottom line, where first bubble appears - where boiling starts)
state variables are properties used to describe the state of a substance, including pressure, temperature, amount of substance and volume. Can give the perfect gas law.
Real gases deviate from ideal gas behaviour at low temperatures and high pressures - when intermolecular forces are non-zero. This is because at low temperatures, molecules no longer have sufficient kinetic energy to overcome their attractive intermolecular forces which means Z<1. At high pressures, molecules are forced closer together, volume of the molecules becomes significant and is no longer negligible, repulsive intermolecular forces dominate and Z>1.
Dipoles - difference in electronegativity between two atoms creating a dipole moment.
Multipoles - combination of dipoles if more than one present (total overall dipole moment?)
Polarizability - how easily a molecule is distorted by a dipole moment/electric field. Larger molecules have greater electron density so are more polarizable.
How polar a molecule is - depends on the difference in electronegativity of the atoms.
At a certain pressure and temperature, the state with the lowest gibbs free energy is the most thermodynamically stable and has the least chemical potential. Melting occurs when the transition from solid to liquid produces a negative deltaG, meaning the liquid is more thermodynamically stable that the solid.
Tetrahedral sites: 3 spheres directly on top of 1 sphere.
Octahedral sites: 3 spheres on top of 3 spheres with the gap between spheres aligning to be on top of each other.
Simple cubic: atoms only at cube verticies, coordination number of 6
Body centered cubic: atoms at cube corners and 1 at cube centre, coordination number of 8
Face centred cubic: atom at each corner of cube and in centre of each face, coordination number of 12
Explain why surface tension arises
Surface tension occurs because molecules at the top of a liquid experience unequal intermolecular forces - pulled down by the bulk below but only hit by gas molecules at top. Surface tension designed to minimise surface area: volume ratio.
All colligative properties. Osmotic pressure is the pressure needed to stop osmosis, boiling point elevation occurs because molecules in the liquid get in the way of the molecules boiling off to gas, freezing point depression occurs because impure molecules get in the way of the regular layers that forms when a solid freezes.
Simple distillation - good for when two mixtures have very different boiling points. One (more volatile) boils before the other, is condensed and collected and can be separated.
Fractional distillation - occurs when two mixtures have similar boiling points. Uses a fractional column and creates many small vapour-liquid equilibrium points. Boils, rises up fractioning column, condenses to a purer mixture and process repeats.
Azeotropes are mixtures that boil at a constant temperature and the vapour and liquid states have the same composition. Cannot be separated using simple or fractional distillation.