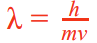Particles - Quantum phenomena
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
Photoelectric effect
Photoelectrons are emitted from the surface of a metal after light above a certain frequency is shone on it
Threshold frequency
The minimum frequency of photons required for photoelectrons to be emitted from the surface of a metal plate through the photoelectric effect
How to increase number of photoelectrons emitted per second
When frequency is above threshold, light intensity can be increased to increase photoelectrons per second
Photoelectron only emitted if frequency above threshold
Work function
Minimum energy required for electrons to be emitted from the surface of the metal
Photon energy directly proportional to frequency
Stopping potential
Potential difference needed to apply across the metal to stop the photoelectrons with maximum kinetic energy
Ek(max) = eVs
e = charge of electron
Vs = stopping potential
Fluorescent tube production of light explanation
This voltage accelerates free electrons through the tube, which collide with the mercury atoms causing them to become ionised, releasing more free electrons. The free electrons collide with the mercury atoms, causing them to become excited. When they de-excite they release photons, most of which are in the UV range. The (phosphorous) fluorescent coating on the inside of the tube, absorbs these UV photons and therefore electrons in the atoms of the coating become excited and de-excite releasing photons of visible light
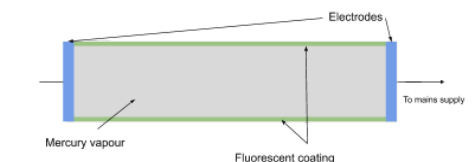
Use of light from fluorescent tube
Pass visible light produced through diffraction grating/prism to form a line spectrum
Line spectrum
Each line represents different wavelength of light.
Only discrete values of wavelength so only photon energies emitted from fluorescent tube shown, thus evidence electrons can only transition between discrete energy levels
Line absorption spectrum
Produced via passing white light through cooled gas
Continuous spectrum of all possible wavelengths of light, with black lines at certain wavelengths (represent possible differences in energy levels since atoms in gas can only absorb photons of energy = difference between two energy levels)
Wave-particle duality examples (brief)
Wave: Diffraction (forms concentric rings), interference, polarisation, refraction
Particle: Photoelectric effect, collisions with other particles (annihilation etc), deflection by electromagnetic field
De broglie wavelength equation explanation of electron diffraction patterns
When momentum increases, wavelength decreases hence amount of diffraction decreases, so concentric rings of interference pattern become closer and vice versa