Module 1 Drug Metabolism: Purpose, Pharmacological Consequences & Metabolic Reactions and Enzymes
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
54 Terms
What is drug metabolism?
It is the process by which drugs or any foreign substances (xenobiotics) are chemically altered within a living organism.
What is the primary function of metabolism?
The primary function is to convert lipophilic molecules to its hydrophilic form, so drugs are able to be excreted from the blood —> urine or bile.
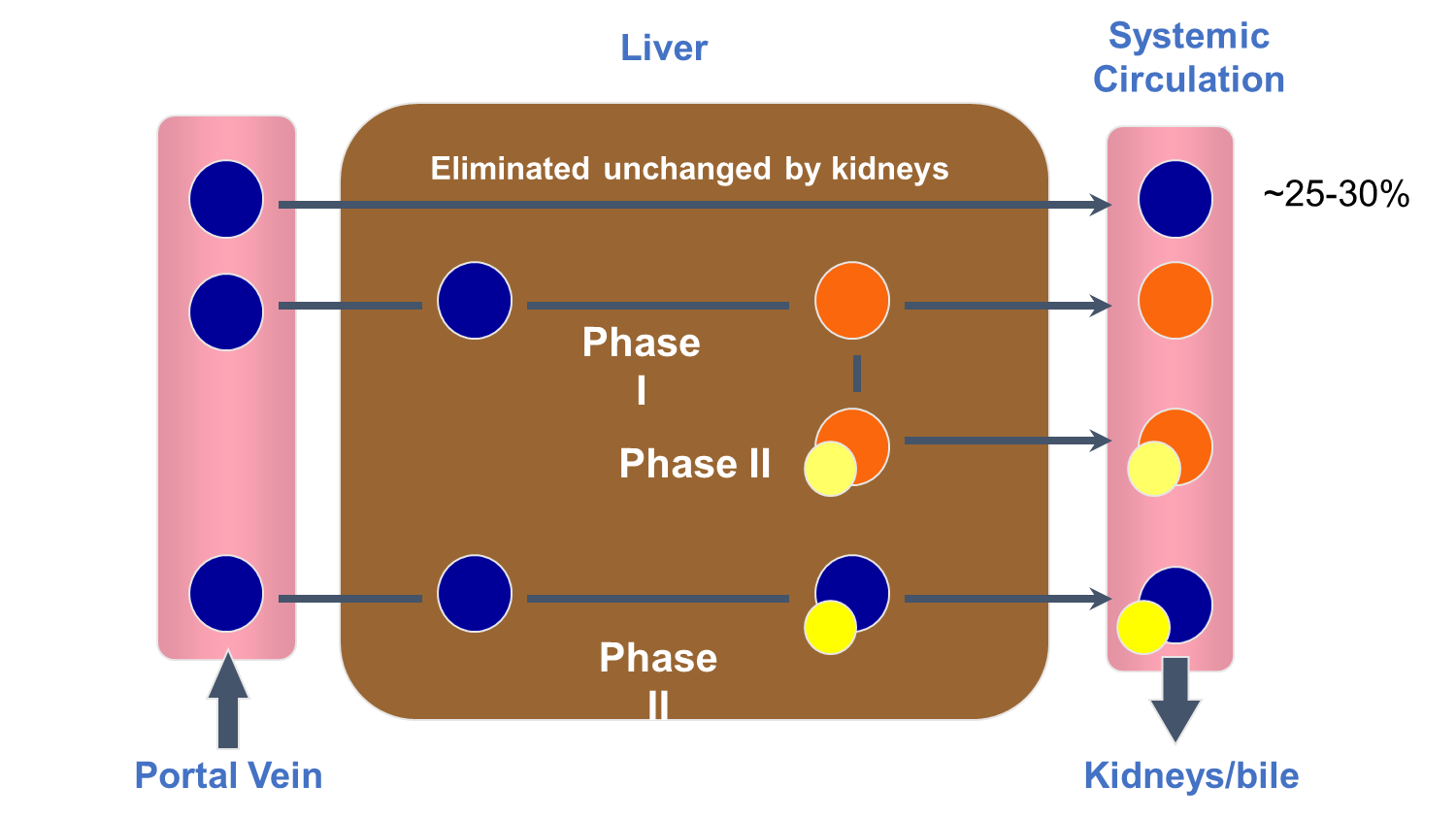
Where does drug metabolism occur the most in the body?
What is another important metabolic site?
LIVER!
also important metabolic site: GI tract (gut microbiome)
True or false: Some enzymes are dedicated to xenobiotics, while other have roles for normal physiological processes
True!
CP450 has both xenobiotic and physiological process functions
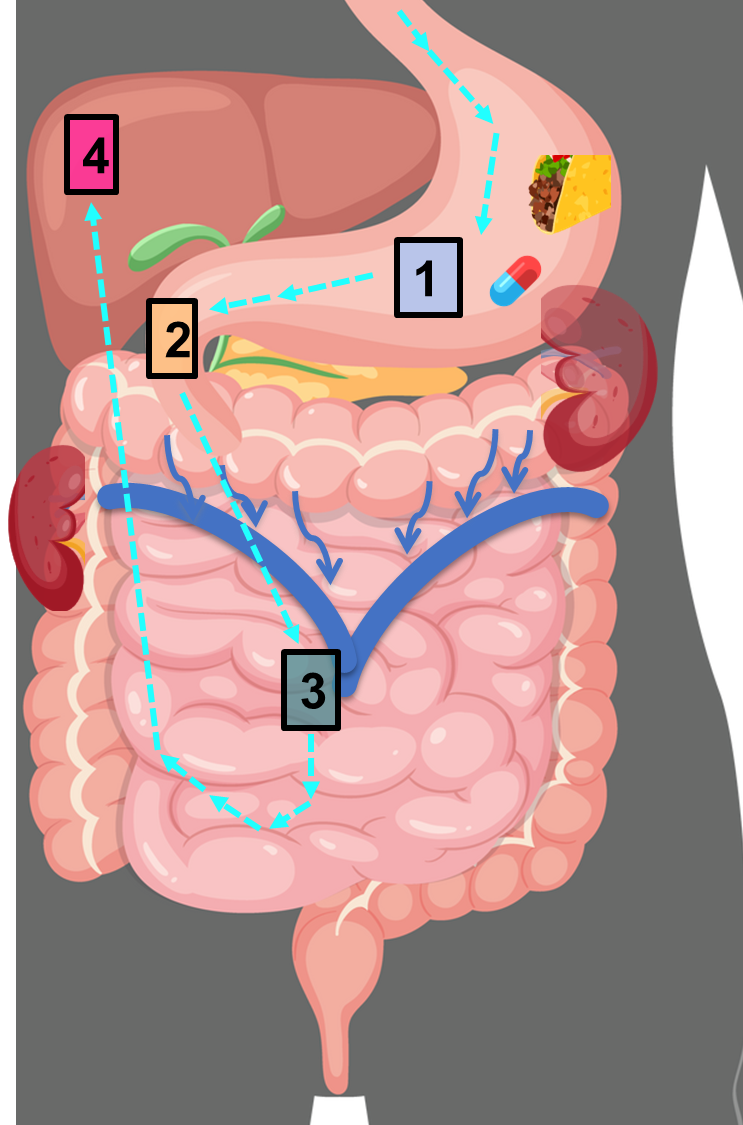
List the following steps of drug metabolism in order:
a. blood from GI tract is collected into portal vein and transported to liver
b. drug is orally ingested
c. the products are excreted by the liver into bile or systemic circulation for renal elimination
d. drug is absorbed into GI tract
e. liver metabolizes the drug
b → d → a → e → c
Match the following drug metabolism process to its definition:
Drug is conjugated in the liver → excreted via bile that goes to the gut → drug is deconjugated in the gut and parent drug is reabsorbed.
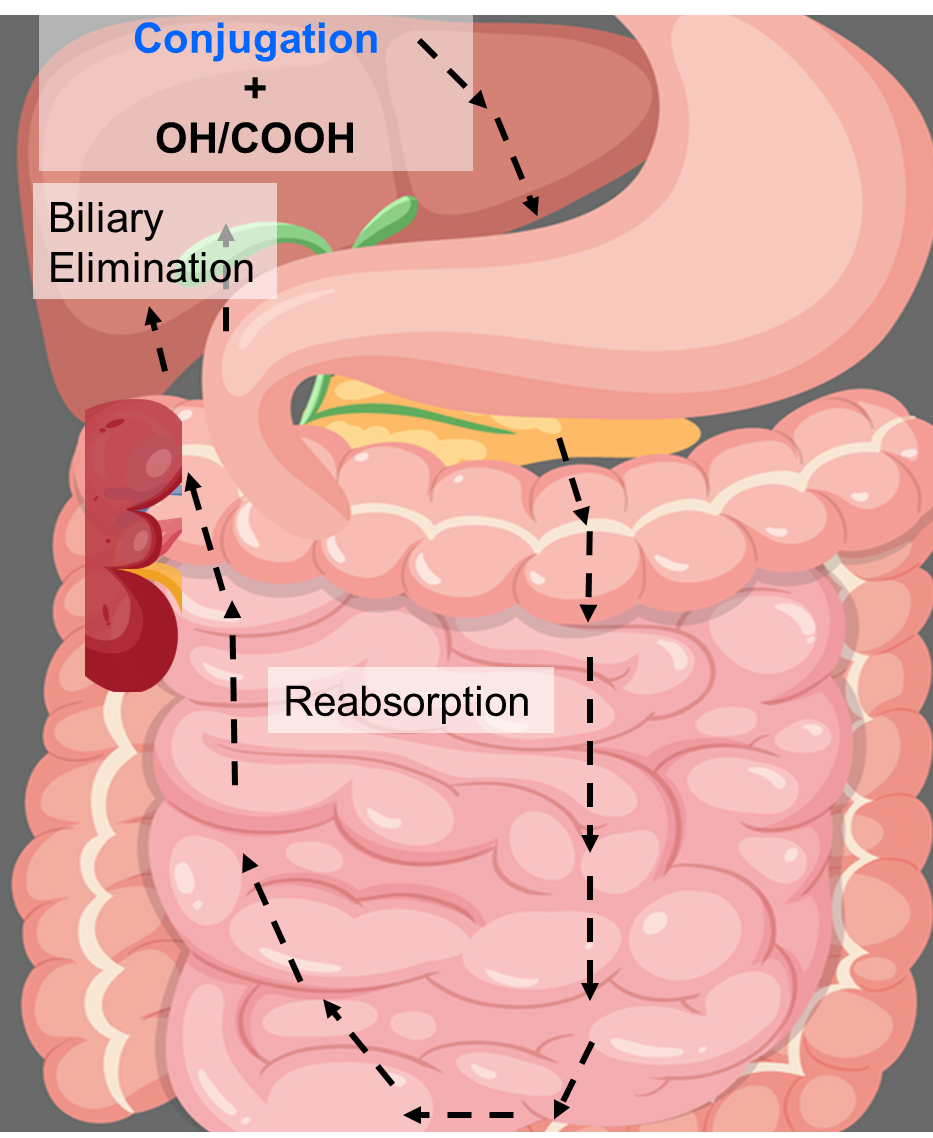
a. Enterohepatic Recirculation
b. First-Pass Effect
a. Enterohepatic Recirculation
Match the following drug metabolism process to its definition:
Extensive metabolism of some drugs during absorption and/or first pass through the liver
a. Enterohepatic Recirculation
b. First-Pass Effect
b. First-Pass Effect
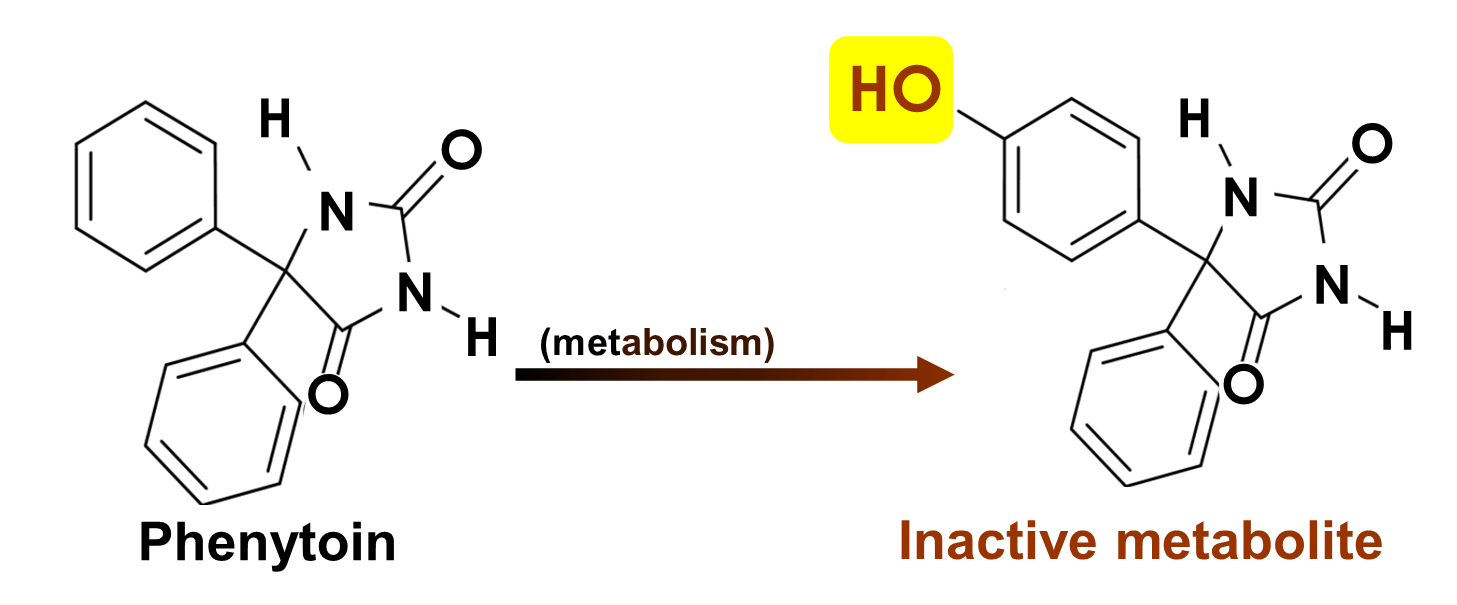
Phenytoin is an example of what kind of consequence of metabolism?
a. pharmacological deactivation
b. metabolites remain active
c. metabolic activation
d. change in TYPE of pharmacological response
e. toxicological activation
a. pharmacological deactivation
most common consequence
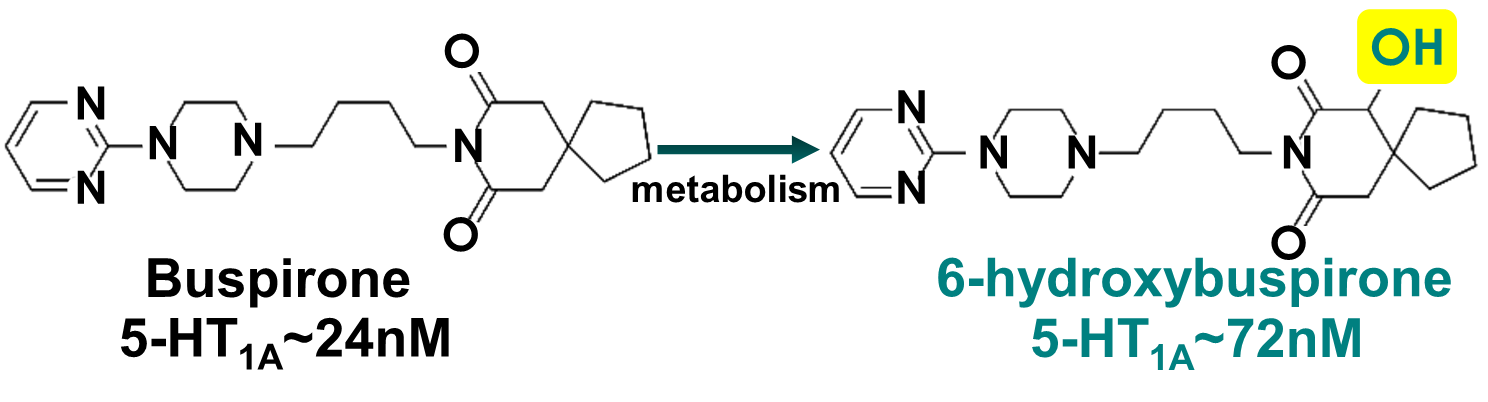
Buspirone is an example of what kind of consequence of drug metabolism?
Hint: the metabolic transformation does not cause loss of activity → metabolite may become stronger or weaker than parent drug
a. pharmacological deactivation
b. metabolites remain active
c. metabolic activation
d. change in TYPE of pharmacological response
e. toxicological activation
b. metabolites remain active
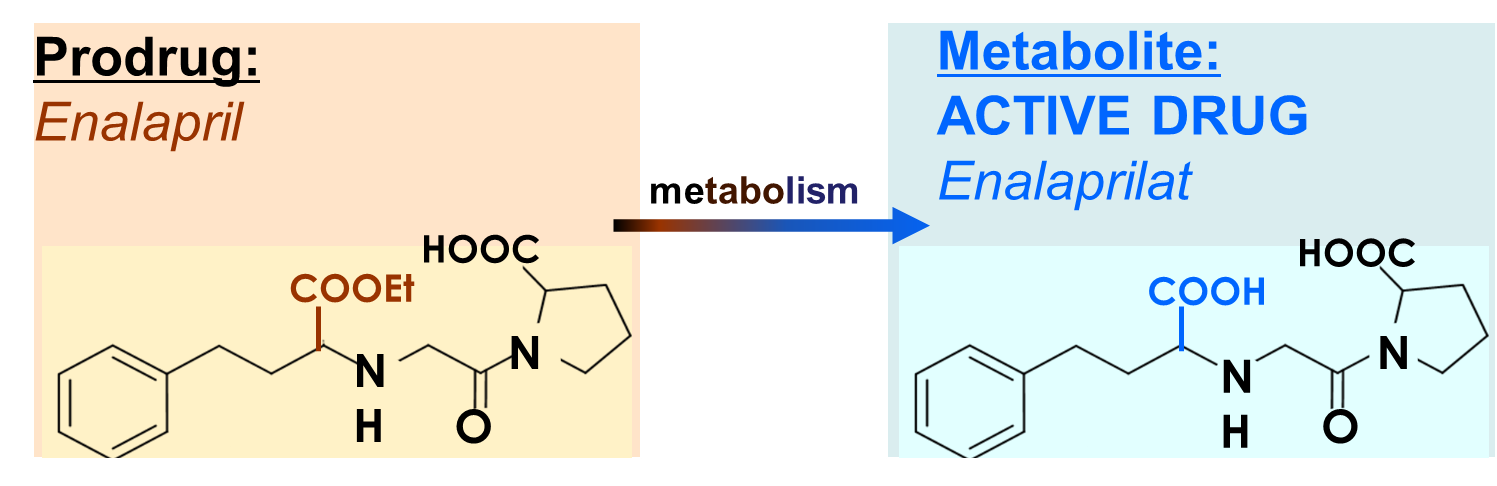
Enalapril (a prodrug) is an example of what kind of consequence of drug metabolism?
a. pharmacological deactivation
b. metabolites remain active
c. metabolic activation
d. change in TYPE of pharmacological response
e. toxicological activation
c. metabolic activation
parent drug is not active → activated by metabolic transformation
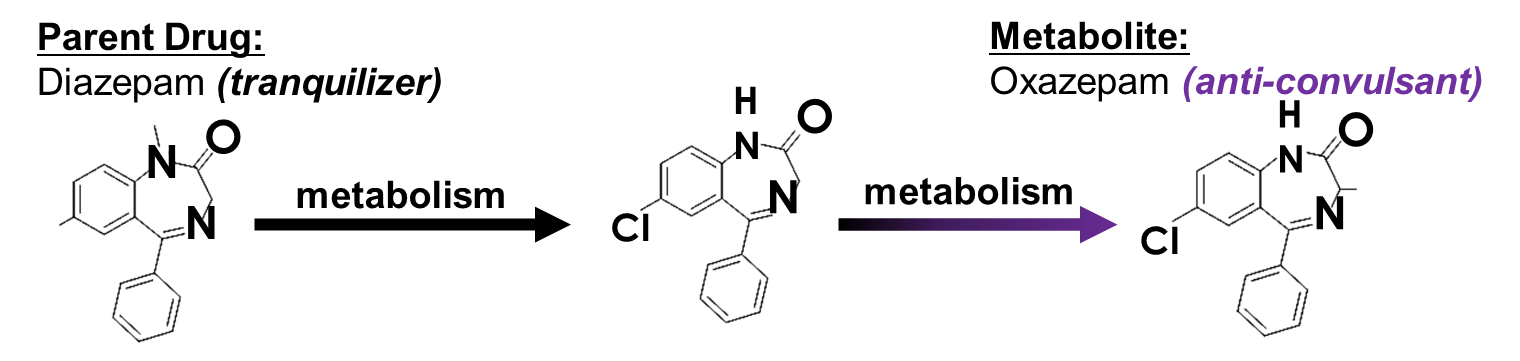
Diazepam (tranquilizer) is an example of what kind of consequence of drug metabolism?
Hint: it is a rare consequence
a. pharmacological deactivation
b. metabolites remain active
c. metabolic activation
d. change in TYPE of pharmacological response
e. toxicological activation
d. change in TYPE of pharmacological response
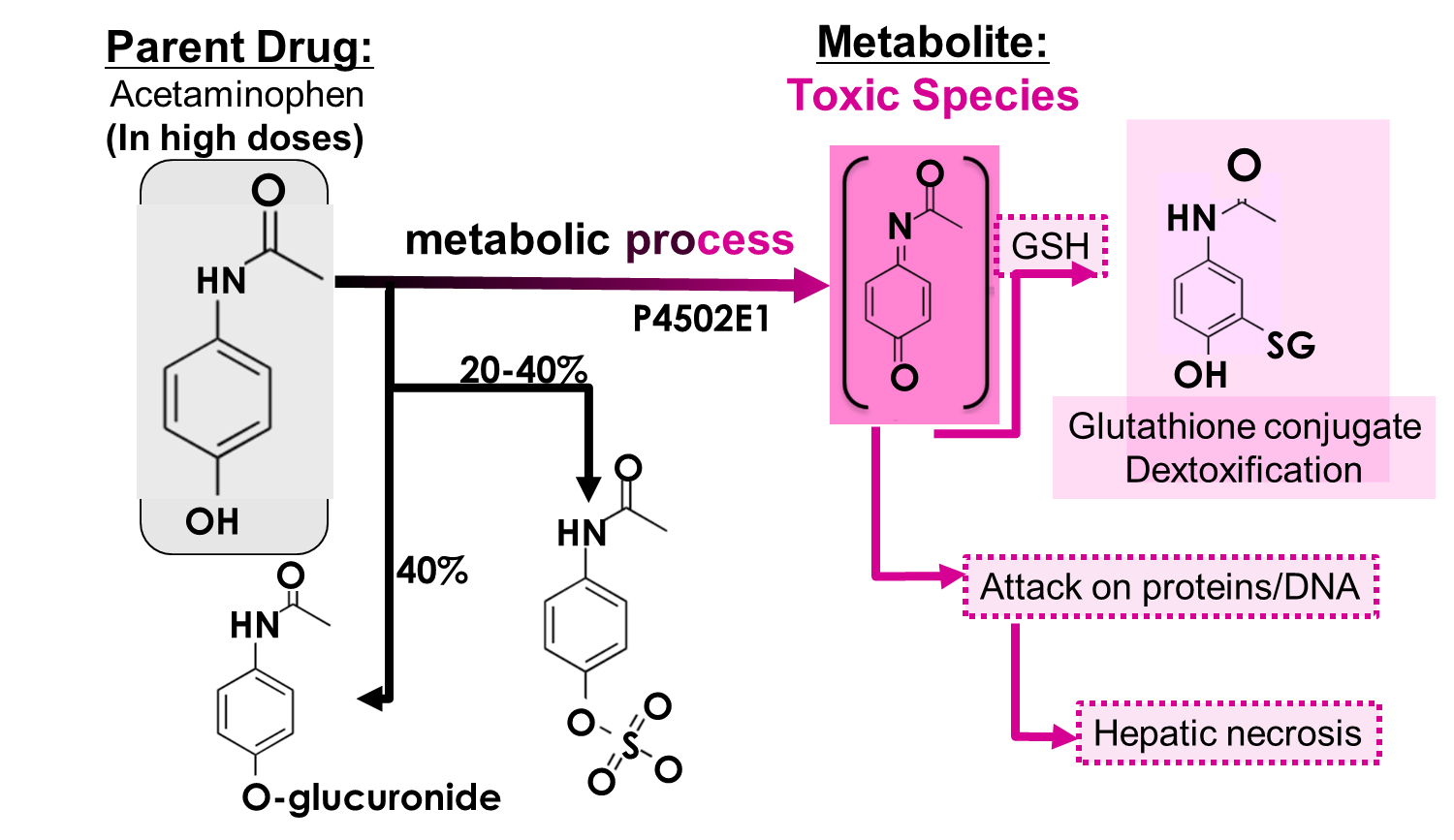
High doses of acetaminophen is an example of what kind of consequence of drug metabolism?
Hint: this is a highly undesirable phenomenon
a. pharmacological deactivation
b. metabolites remain active
c. metabolic activation
d. change in TYPE of pharmacological response
e. toxicological activation
e. toxicological activation
metabolic transformation converts drugs into toxic species
Match the term to its definition:
Also known as Functionalization
prepares drug for further conjugation
adds/expose functional groups
a. Phase I
b. Phase II
a. Phase I
Match the term to its definition:
Also known as Conjugation
can occur without Phase I
transferase enzyme adds a molecule (conjugate) to the drug or its metabolite.
a. Phase I
b. Phase II
b. Phase II
Select which of the following is a reaction in Phase I?
a. Oxidation
b. Acetylation
c. Reduction
d. Methylation
e. Hydrolysis
f. Glutathione
a. Oxidation
c. Reduction
e. Hydrolysis
Select which of the following is a reaction in Phase II?
a. Oxidation
b. Acetylation
c. Reduction
d. Methylation
e. Hydrolysis
f. Glutathione
b. Acetylation
d. Methylation
f. Glutathione
As well as glucuronic acid, sulfuric acid, amino acid
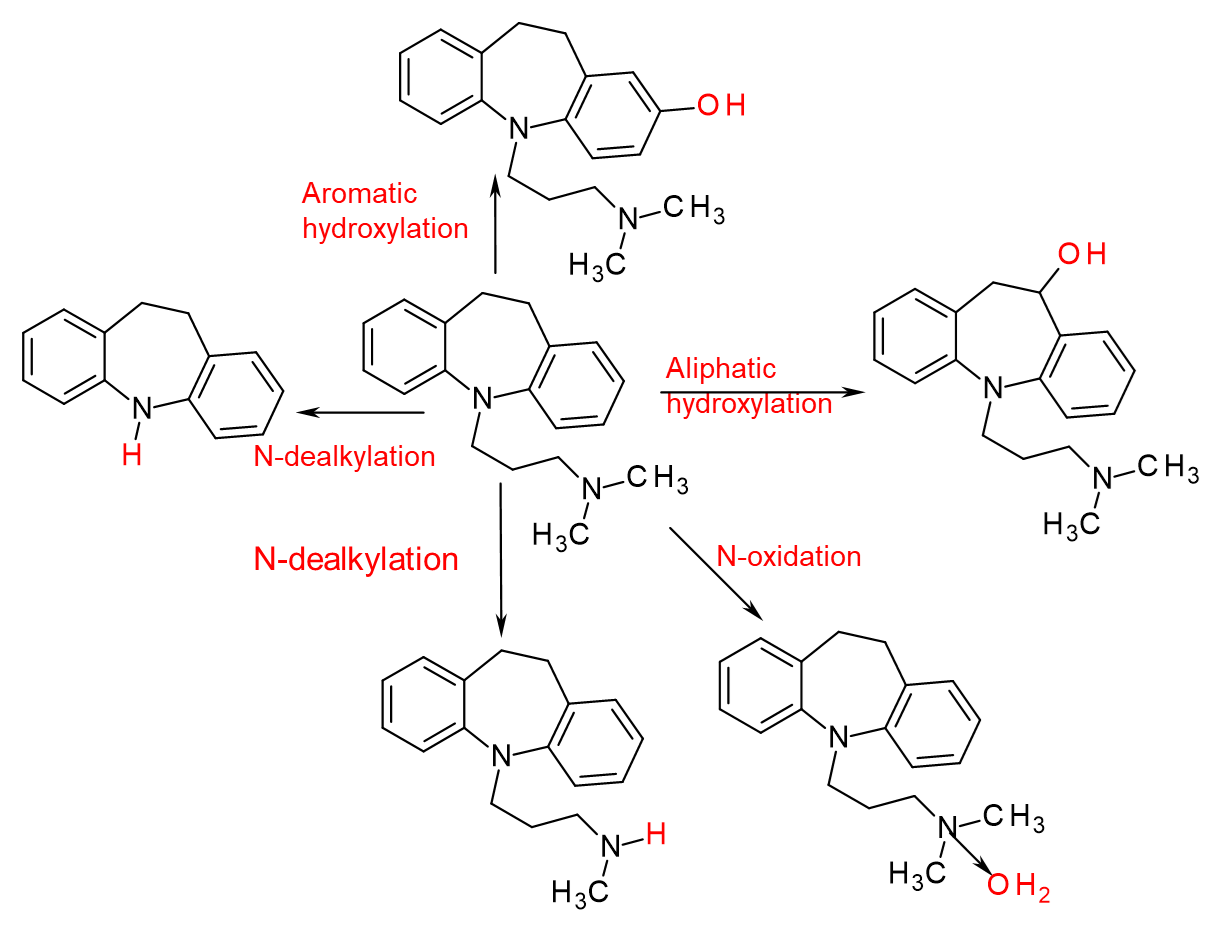
Hydroxylation, deamination, and dealkylation, are what kind of reactions in Phase I?
a. Oxidation
b. Reduction
c. Hydrolysis
a. Oxidation
Oxidation is the addition of ___ and/or the removal of ___
O2, Hydrogen


Reduction is the addition of ___ and/or the removal of ____ or ___ groups
H, Oxygen, NO2 (nitro)
Aldehyde → Alcohol
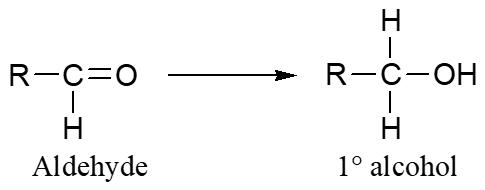
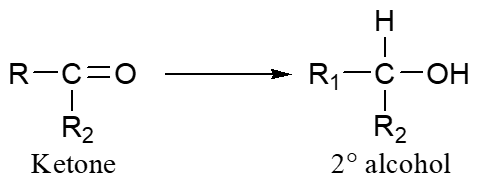
Ketone → Alcohol
Nitro group (NO2) → Amine (NH2)
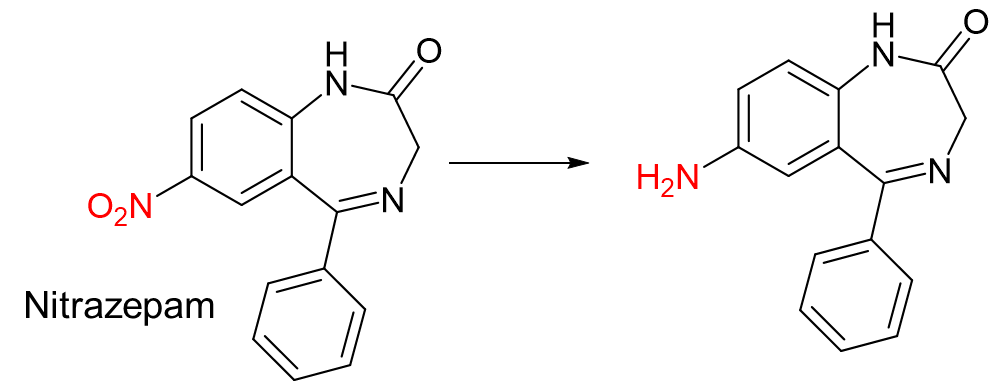
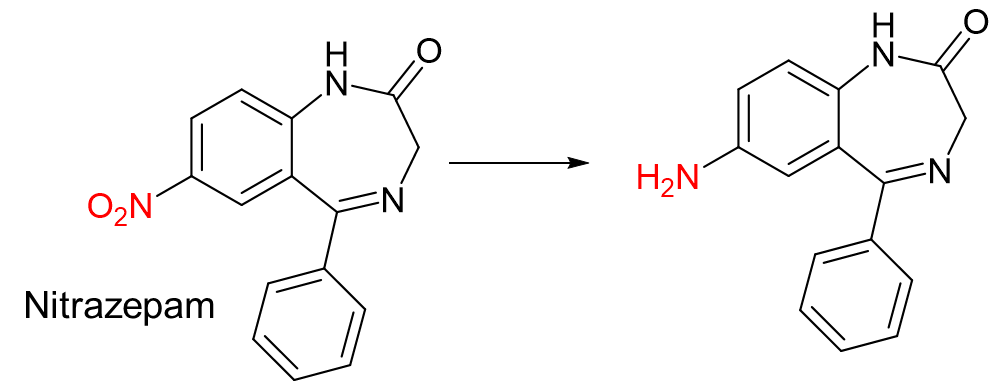
This reaction is an example of…
a. Oxidation
b. Reduction
c. Hydrolysis
b. Reduction
Hydrolysis is the addition of ____ with the breakdown of the molecule in blood plasma and liver.
H2O
Ester → Alcohol
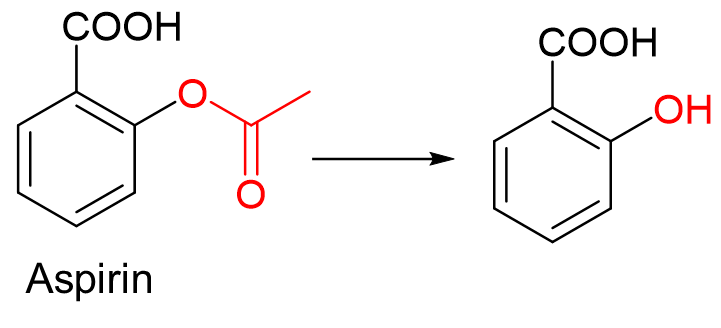
Amide → Carboxylic acid
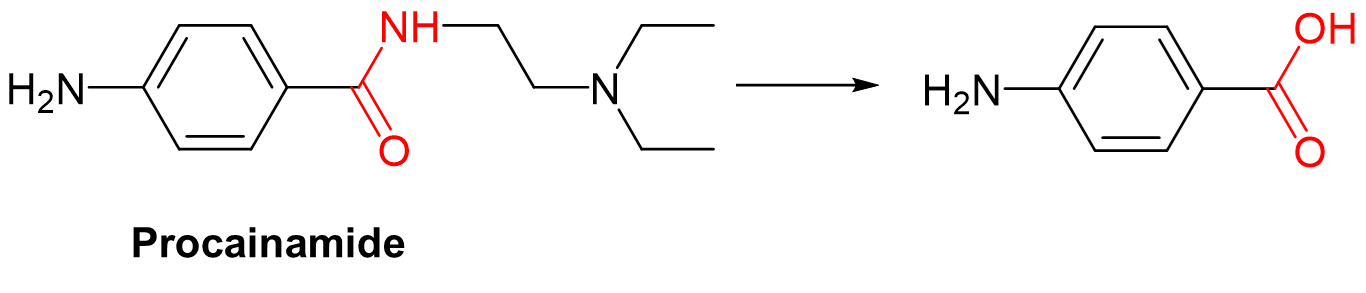
True or False: All drugs must be metabolized.
False! There are some drugs that do need to be metabolized (already in hydrophilic species)
What is the major enzyme that catalyze phase I metabolism?
a. UDP-glucuronosyl transferase
b. Cytochrome P450
b. Cytochrome P450
What is the major enzyme that catalyze phase II metabolism?
a. UDP-glucuronosyl transferase
b. Cytochrome P450
a. UDP-glucuronosyl transferase
What does the P450 active site consist of?
Hint: it contains a heme (iron, Fe) in a hydrophobic pocket that is bonded to 6 ligands
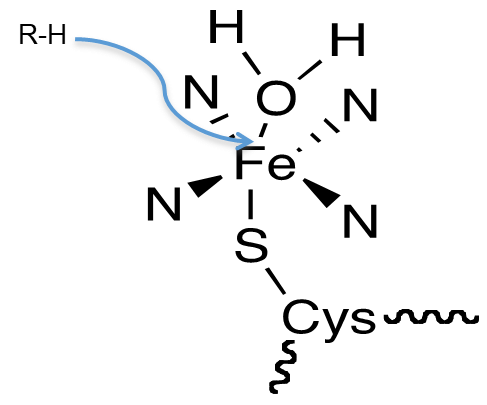
4 ligands → 4 pyrrole (N) rings
5th ligand → thiolate (S) anion
6th ligand → H2O molecule
What is the general P450 reaction?
has 2 parts
RH + O2 + NADPH + H+ → ROH + NADP+ + H2O
Incorporates 1 atom of Oxygen into the substrate with the enzyme monooxygenase.
NADPH serves as an electron donor
Which of the following statements regarding drug metabolism is CORRECT?
I. Phase I metabolites are always pharmacologically inactive
II. Phase I metabolism is prerequisite for the Phase II metabolism
III. Some drugs are not active unless metabolized inside the body
A.I, only
B. III, only
C.I and II, only
D.II and III, only
E.All of the above
B. III, only
Why is NADPH-cytochrome P450 reductase important in the general P450 reaction?
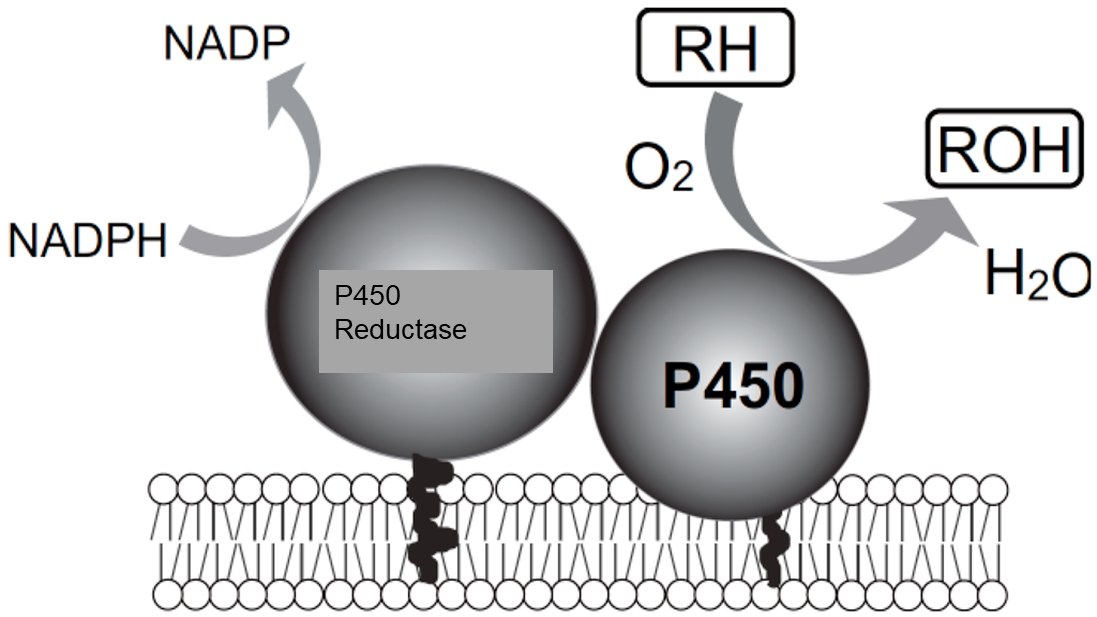
P450 reductase is an electron carrier
NADPH → NADP+ reaction gives 2 electrons
P450 reaction only NEEDS 1 electron
main purpose: P450 reductase gives P450 1 electron at a time
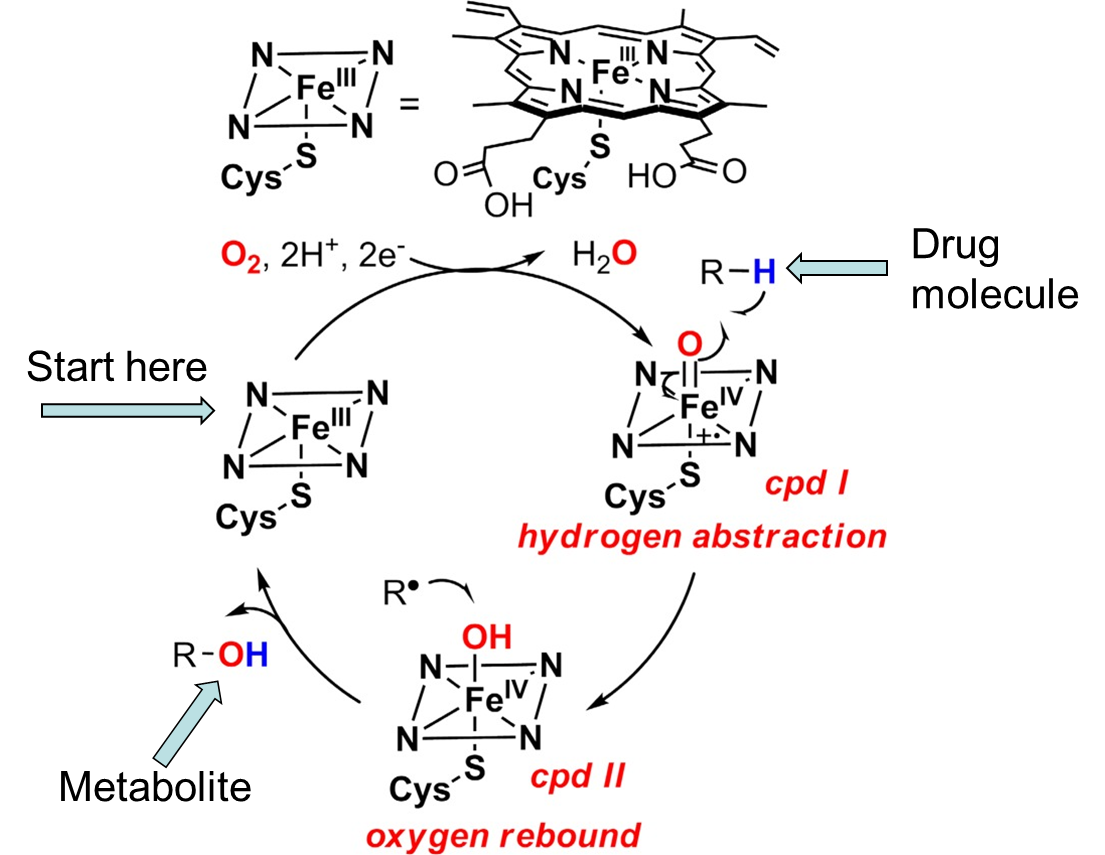
list the steps of the catalytic cycle in order:
a. oxygen activation > drug binds to enzyme > substrate oxidation
b. substrate oxidation > drug binds to enzyme > oxygen activation
c. drug binds to enzyme > oxygen activation > substrate oxidation
c. drug binds to enzyme > oxygen activation > substrate oxidation
Radical (R) → grabs H from drug molecule → forms Metabolite (R-OH)
Fe IV goes back to Fe III
Which of the following is NOT a reaction that is catalyzed by P450?
a. Aliphatic hydroxylation
b. Allylic Hydroxylation
c. Benzylic Hydroxylation
d. Aromatic Hydroxylation
e. Epoxidation
f. Heteroatom (N,O, S) dealkylation
g. Heteroatom hydroxylation
h. 2-Electron oxidation of hydroquinones, catechols, 4-alkylphenols, 4-aminophenols --> Quinoid
i. None of the above
i. None of the above
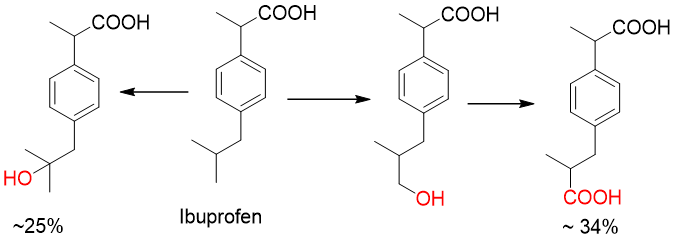
Which reaction is shown above?
a. Aliphatic hydroxylation
b. Allylic Hydroxylation
c. Benzylic Hydroxylation
d. Aromatic Hydroxylation
a. Aliphatic hydroxylation
R-H → R-OH
carbon that is oxidized is aliphatic

Most stable radical: ___ reactive to hydroxylation
Least stable radical: ___ reactive to hydroxylation
more, least
True or False: Allylic is more susceptible than Aliphatic towards hydroxylation.
Aliphatic:
Allylic:
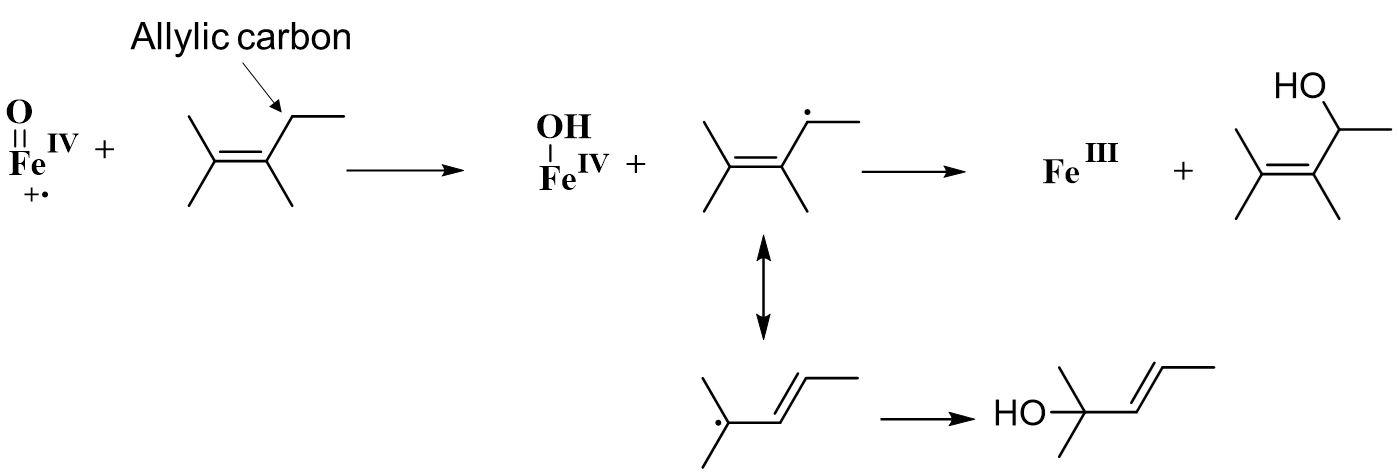
True!
Why?
The radical intermediate is stabilized by the pi system of the double bond.
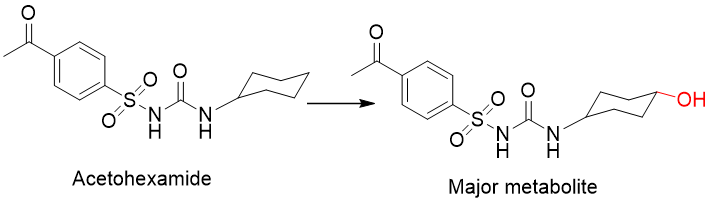
Example: Hexobarbital
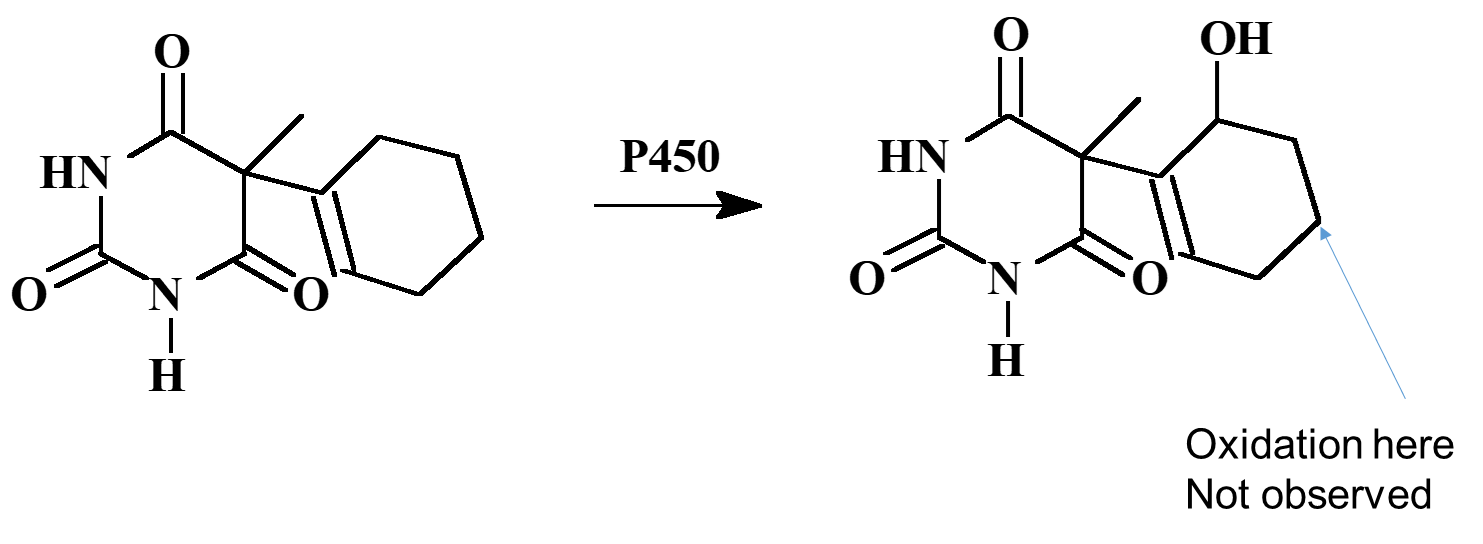
Tolbutamide (hypoglycemic for diabetes)
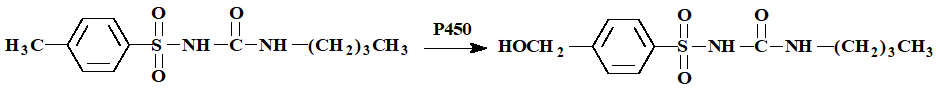
Benzylic hydroxylation is similar to what kind of hydroxylation?
a. allylic
b. aliphatic
c. benzylic
a. allylic
Benzylic hydroxylation —> radical intermediate is stabilized by the aromatic ring
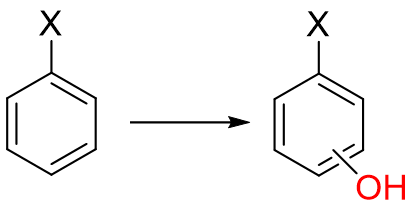
In Aromatic hydroxylation, what is the role of X?
a. enhance oxidation rate and direct substitution to ortho/para position
b. slow down or prevent aromatic hydroxylation and direct to meta position
c. position and rate of hydroxylation
c. position and rate of hydroxylation
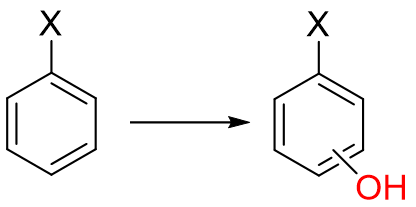
In Aromatic hydroxylation, what is the role of EDG (electron donating groups)
OH, R, NH2
a. enhance oxidation rate and direct substitution to ortho/para position
b. slow down or prevent aromatic hydroxylation and direct to meta position
c. position and rate of hydroxylation
a. enhance oxidation rate and direct substitution to ortho/para position
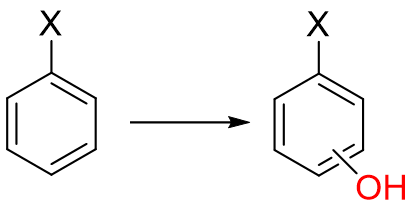
In Aromatic hydroxylation, what is the role of EWG (electron withdrawing groups)
NO2, C=O, COOH, +NR3
a. enhance oxidation rate and direct substitution to ortho/para position
b. slow down or prevent aromatic hydroxylation and direct to meta position
c. position and rate of hydroxylation
b. slow down or prevent aromatic hydroxylation and direct to meta position
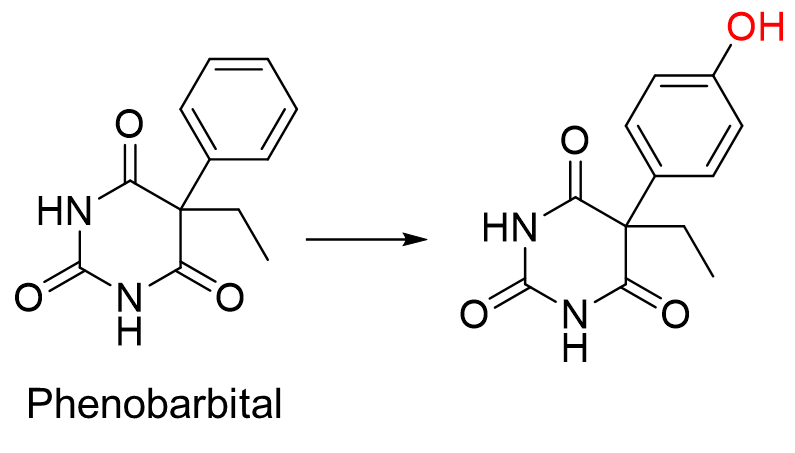
Is this an aromatic or benzylic hydroxylation reaction?
Aromatic hydroxylation
What happens when there is a drug with 2 aromatic rings during aromatic hydroxylation?
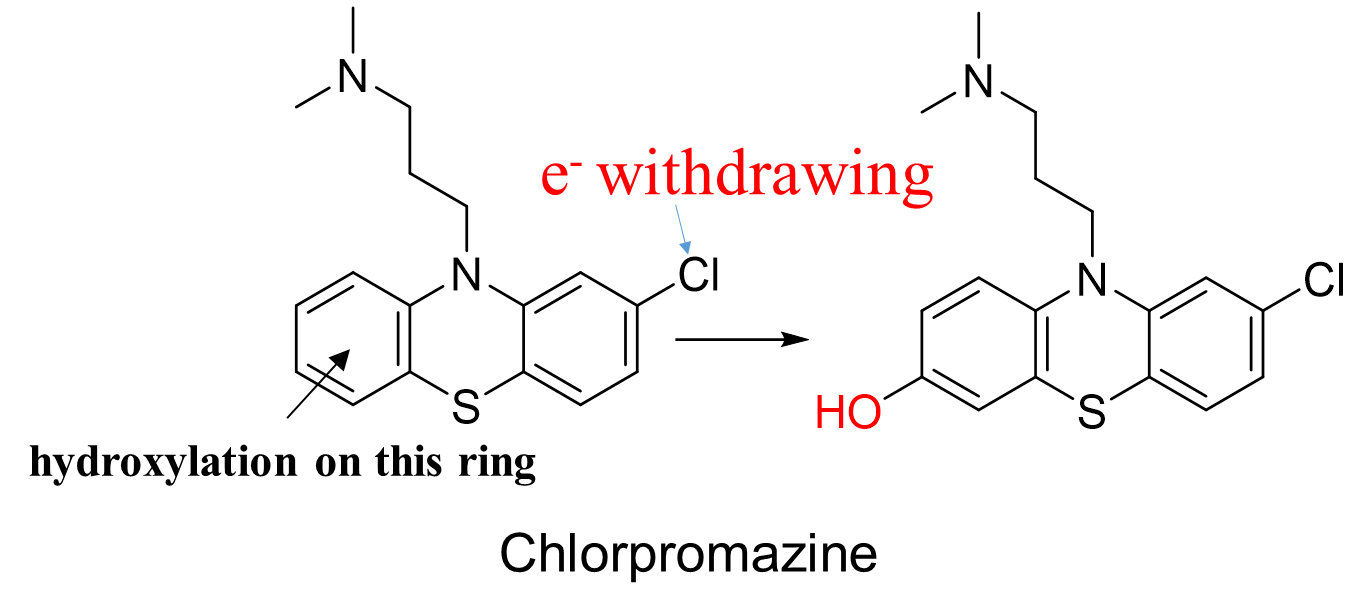
The more e- rich is usually hydroxylated
What if there are other metabolic sites that compete with aromatic hydroxylation?
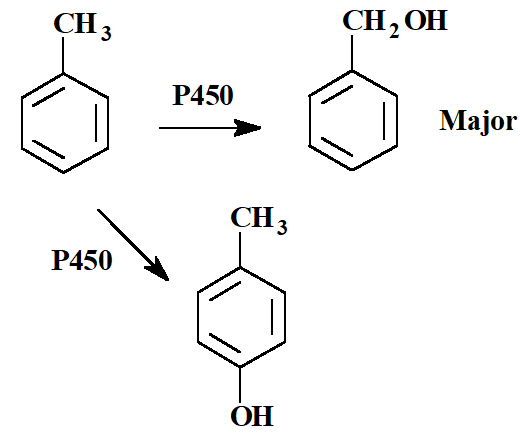
The importance of aromatic hydroxylation decreases because it is difficult to oxidize an aromatic ring
why? → it destroys the aromaticity in the process
If CYP450 catalyzed metabolism of toluene was studied in a buffer prepared with water in which oxygen was substituted with O18 isotope (H2O18) what could be the product of the reaction?
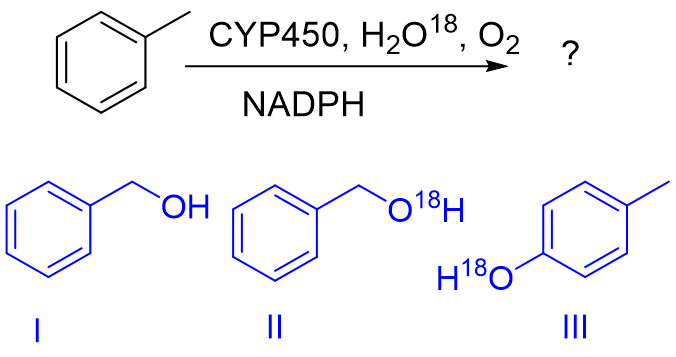
A. I, only
B. II, only
C. I and II
D. II and III
E. All of the above
A. I, only
Explain how epoxidation works.
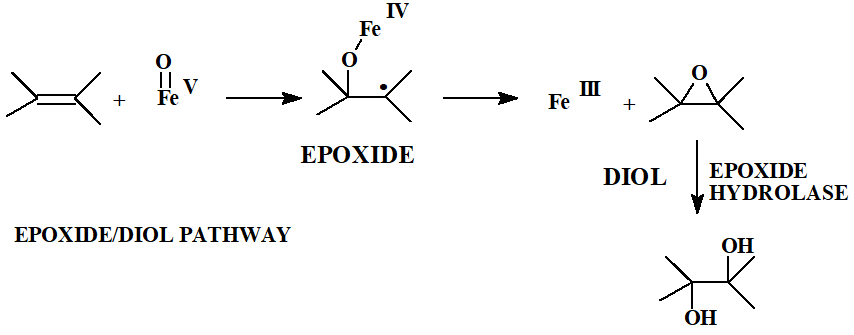
Iron oxo intermediate inserts into pi system of double bond
↓
Radical intermediate snaps shut to give an epoxide
Epoxide hydrolase → phase 1 enzyme that hydrolyses epoxides → forms diols
Heteroatom dealkylation involves N, O, S and has the same mechanism as aliphatic hydroxylation, but what happens in addition?
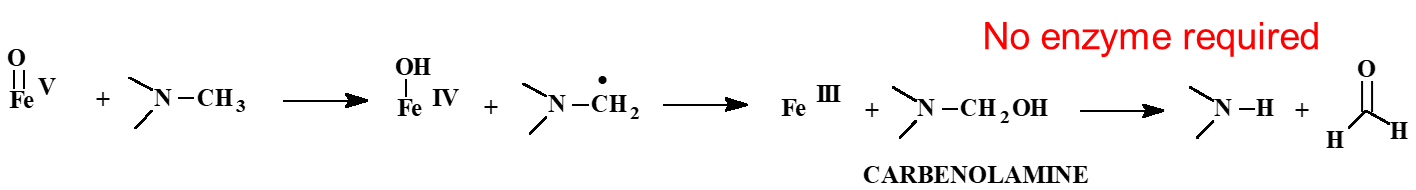
The hydroxylated product (unstable) decomposes non enzymatically (w/o P450) → product is heteroatom dealkylated
NOTE: Carbon next to Nitrogen (𝛼 carbon) MUST HAVE a Hydrogen (𝛼 hydrogen)
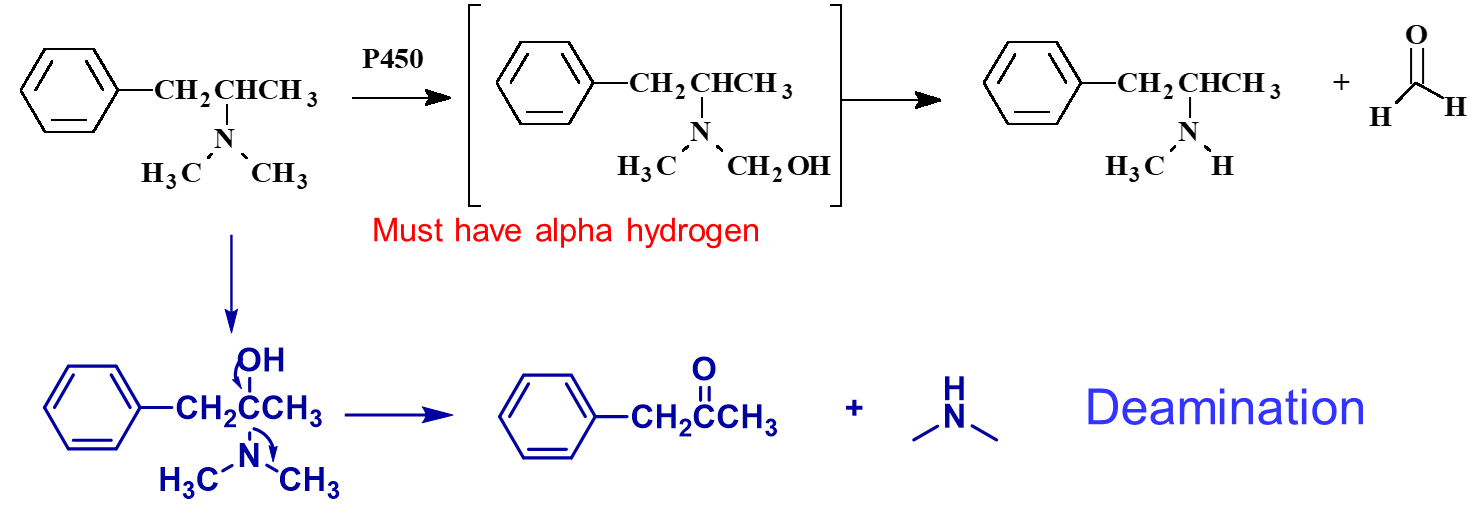
Why doesn’t Finasteride undergo dealkylation?

There is no 𝛼 hydrogen; Finasteride is meant to stop dealkylation
True or False: Heteroatom hydroxylation occurs alongside heteroatom dealkylation.
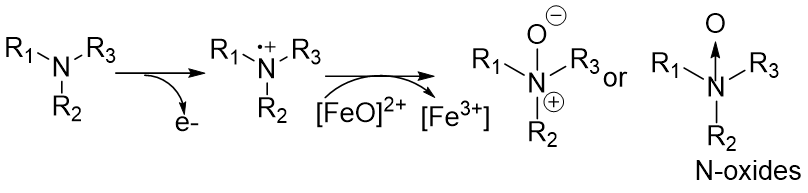
False! Heteroatom hydroxylation occurs when heteroatom dealkylation is NOT POSSIBLE
addition of O directly into heteroatom → forms oxide
2-Electron oxidation of hydroquinones, catechols, 4-alkylphenols, 4-aminophenols → Quinoids
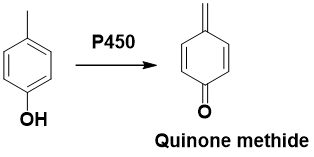
Which position gives Quinone methide?
a. ortho
b. para
b. para
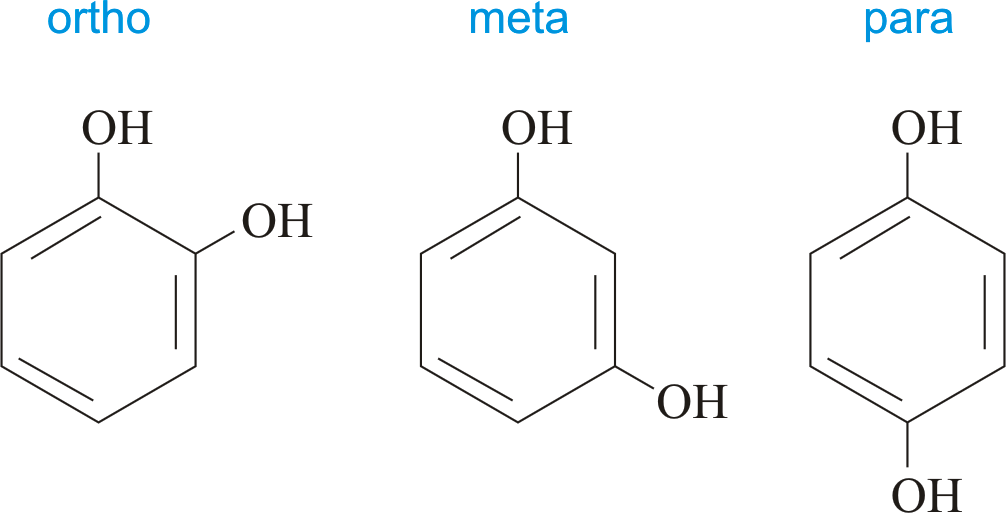
Know this bro
I have looked at this image
2-Electron oxidation of hydroquinones, catechols, 4-alkylphenols, 4-aminophenols → Quinoids
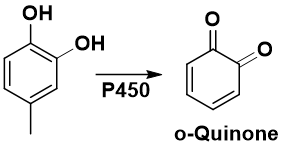
Which position gives Quinone?
a. ortho
b. para
a. ortho
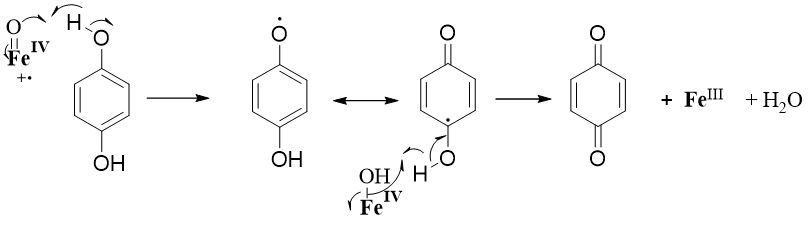
The resulting molecule is what?
Quinone
For naming enzymes, how is an enzyme Family named and how is it defined?
a. labeled with letters
b. labeled with numbers
c. >40% sequence homology
d. >55% sequence homology
b. labeled with numbers
c. >40% sequence homology
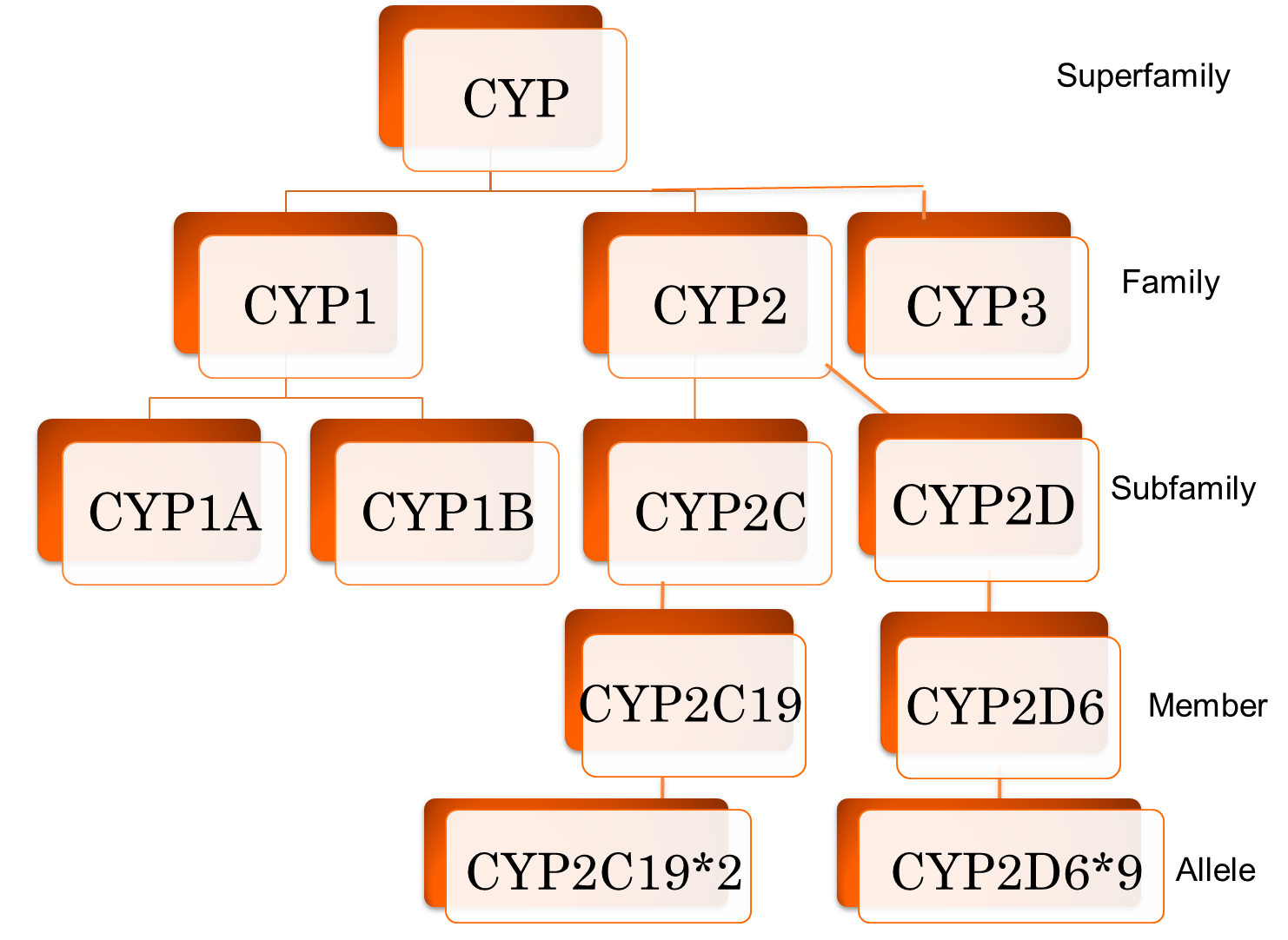
For naming enzymes, how is an enzyme’s subfamily named and how is it defined?
a. labeled with letters
b. labeled with numbers
c. >40% sequence homology
d. >55% sequence homology
a. labeled with letters
d. >55% sequence homology
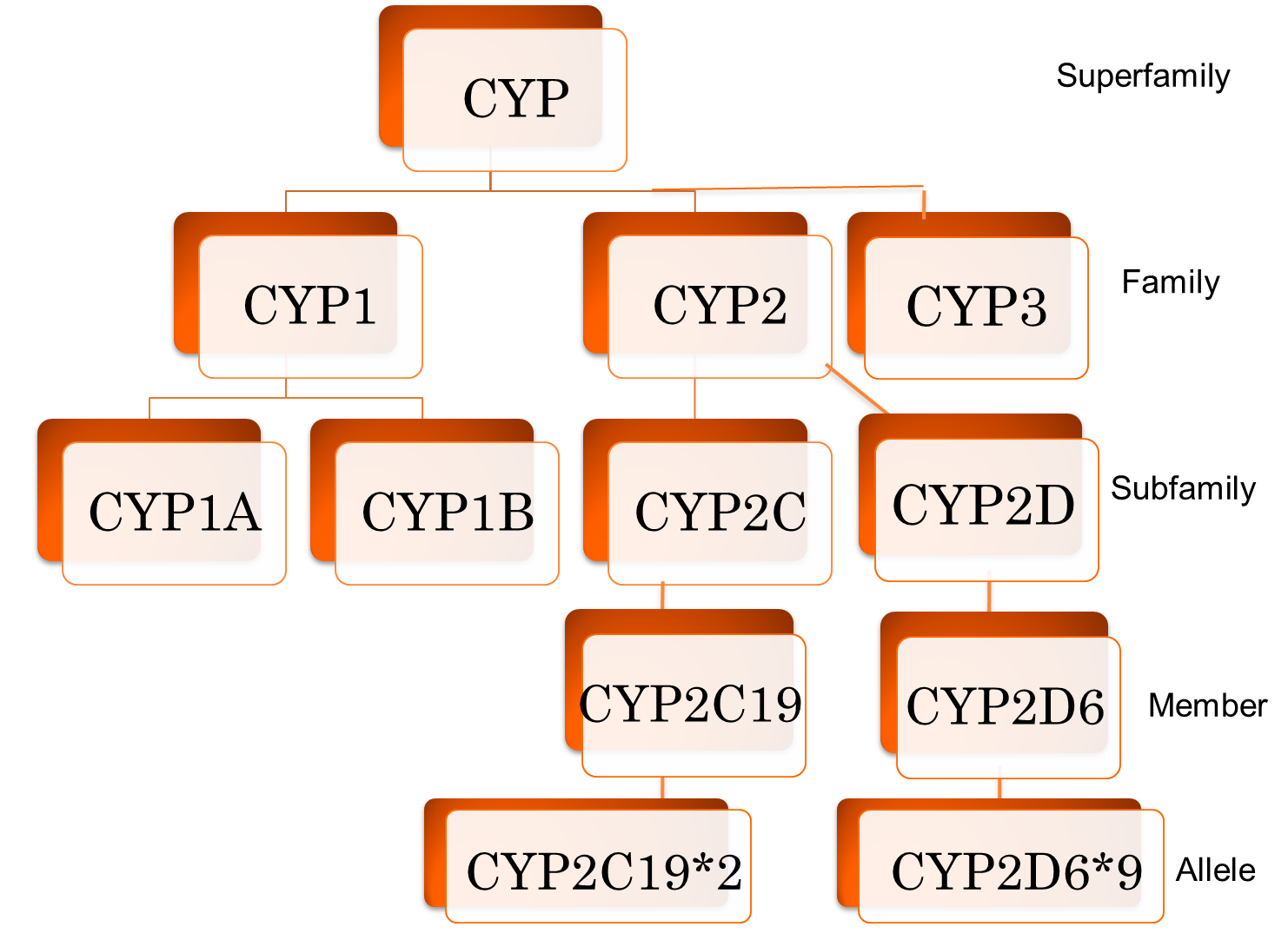
Select all the families that focus on the metabolism of drugs
a. Family 1
b. Family 2
c. Family 3
d. Family 9
e. Family 19
a. Family 1
b. Family 2
c. Family 3
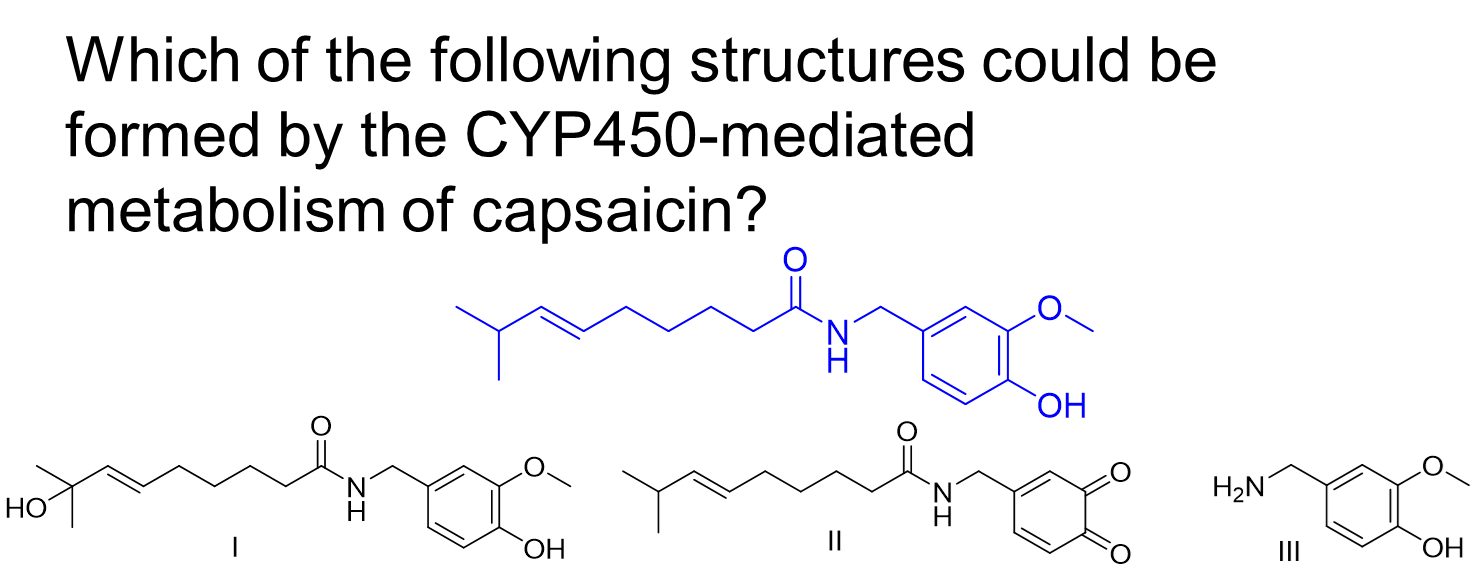
A. I , only
B. III, only
C. I and II, only
D. II and III, only
E. All of the above.
C. I and II, only
Which of the following statements regarding CYP450s is FALSE?
A. CYP3A4 is the most abundant isoform in the liver and contributes the most to metabolism of drugs
B. CYP2D6 contributes to the metabolism of drugs disproportionally to its content in the liver
C. The liver and GI tract are two major sites of CYP450 mediated drug metabolism
D. CYP2D6 shares at least 40% sequence homology with the CYP2C19 isoform
E. The primary role of CYP450s is to metabolize drugs and other environmental agents
E. The primary role of CYP450s is to metabolize drugs and other environmental agents