Chap 19A - Solubility
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
Define saturated solution
Saturated solution (Def.): maximum amount of solute that can be dissolved in a given volume of solvent to form 1dm^3 of a saturated solution at a given temperature
Describe sparingly soluble salts (macroscopic and microscopic changes)
Regardless of how insoluble, a small amount of an ionic salt will always dissolve -> sparingly soluble
When an excess of a sparingly soluble salt is shaken with water, most of the solid remains undissolved -> no significant macroscopic changes
Sub-miscroscopically: Ions from the solution are joining the ionic lattice while ions from the lattice are becoming solvated -> eventually, a saturated solution is formed where the dissolved ions are in dynamic equilibrium with the remaining undissolved solid -> rate of dissolution = rate of precipitation AND concentrations of ions remain constant.
Saturated = equilibrium
Define solubility + units
(Def.): maximum amount of a solute that can be dissolved in a given volume of solvent to form 1 dm3 of a saturated solution at a given temperature
Expressed in mol dm–3, g dm–3, or g per 100 g solvent
How is the solubility of a sparingly soluble salt determined at a given temperature?
At a given temperature, dissolve the salt in deionised water until some undissolved salt is visibly left in the mixture.
Filter the mixture and collect the filtrate.
Pipette a known volume of the filtrate into an evaporating dish of a known mass.
Evaporate to a constant mass.
Weigh the evaporating dish again to calculate the mass of the residue by subtraction.
Divide the mass of the residue by the known volume to obtain the solubility of the sparingly soluble salt (in g cm–3)
Define Ksp
(Def.): Ksp is defined as the product of the concentrations of the constituent ions in a saturated solution of the salt raised to the powers as indicated by the stoichiometric coefficients in the balanced equation for the equilibrium
State dissociation equilibrium equation, constant and units
General dissociation equilibrium equation: AxBy(s) ⇌ xAy+(aq) + yBx–(aq)
Equilibrium constant (Ksp) for sparingly soluble salts = [Ay+]x [Bx–]y
Units of Ksp are (mol dm–3)x+y
NOTE: MUST have state symbols + dissolve 1 mol of compound
When temperature changes, the value of the Ksp will change
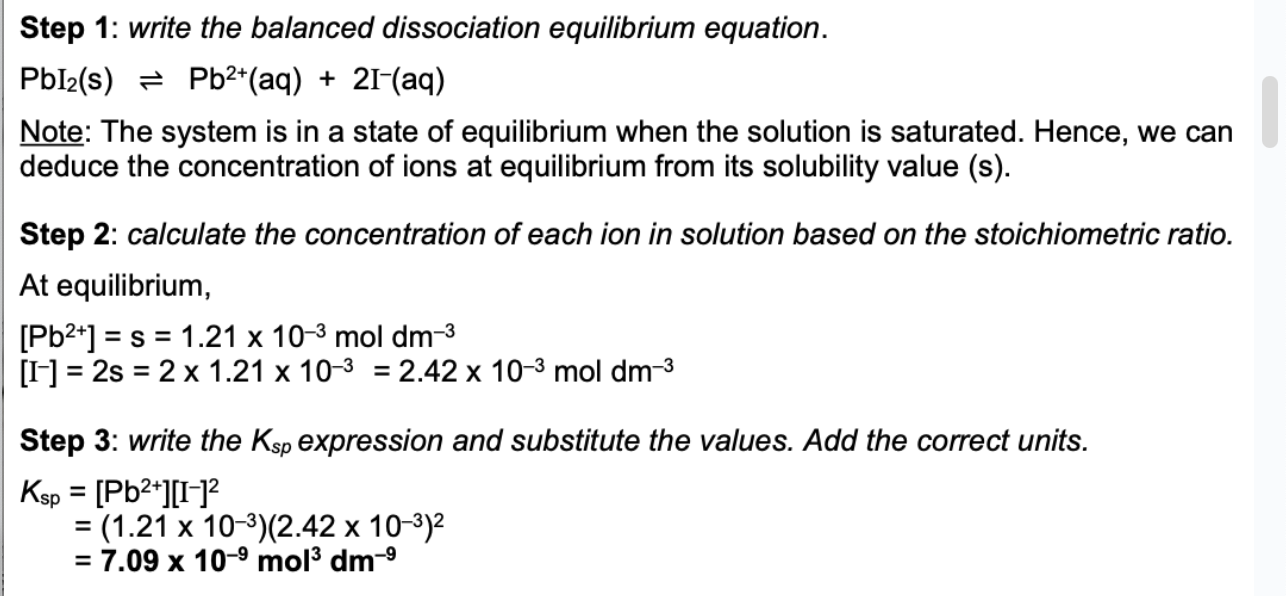
The solubility of PbI2 at 25C is 1.21 × 10^-3 mol dm³. Find solubility product of the salt at this temperature
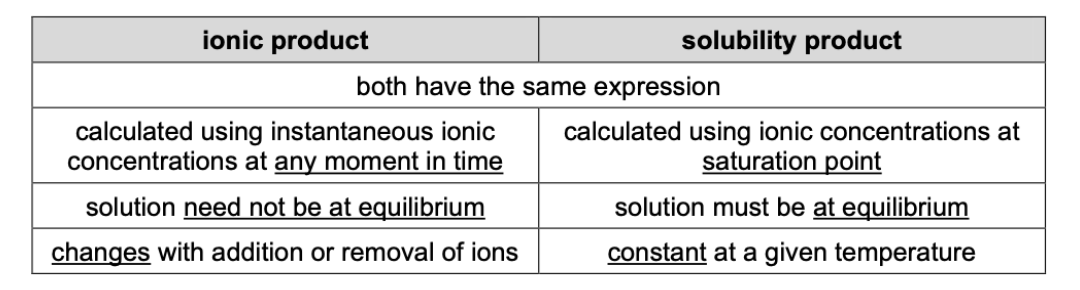
State similarities and differences between ionic product and Ksp
Predict precipitation when ionic product < = > Ksp
ionic product < Ksp | Ionic product = Ksp | Ionic product > Ksp |
|
|
|
What happens with lower Ksp
Lower the Ksp (less soluble) -> more easily it is exceeded by the ionic product + more readily it will precipitate from solution
If units for Ksp are different -> Ksp CANNOT be used to compare solubility of 2 salts -> solubilities have to be calculated
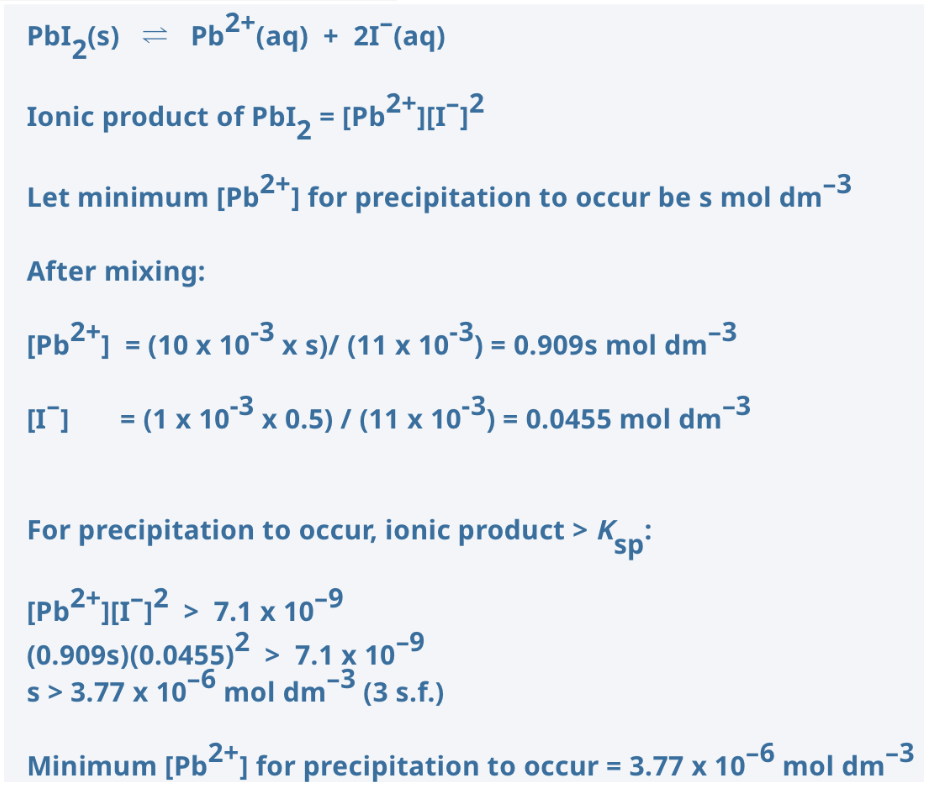
1 cm3 of 0.5 mol dm-3 aqueous KI is added to 10 cm3 of wash water containing Pb2+. What is the minimum [Pb2+] in the wash water that will result in precipitation of yellow PbI2? (Ksp of PbI2 = 7.1 x 10-9 mol3 dm-9)