The Haber process
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Last updated 6:22 PM on 7/27/24
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
1
New cards
What is the equation for the Haber process?
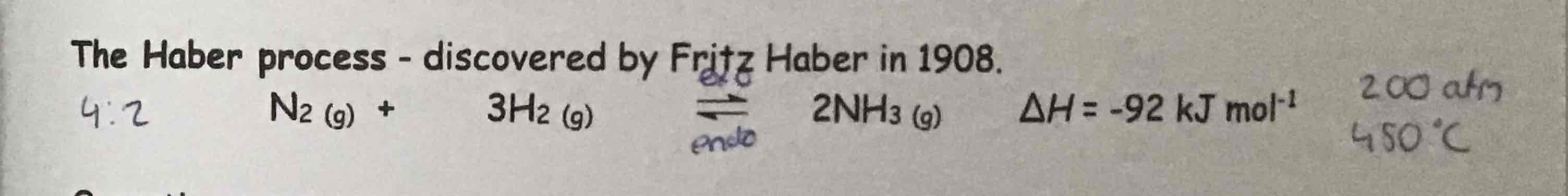
2
New cards
What are the conditions used in the Haber process and why do we use them?
Temperature
450°C
Compromise between yield and rate of reaction
Forward reaction is exo so too high gives us a lower yield of ammonia but too low means slower rate of reaction
High temp= lower equilibrium yield but faster rate of reaction
Pressure
200 atm
Higher pressure gives higher yield of ammonia
Too high of pressure means it’s very expensive so compromise is used
Catalyst
Phosphoric acid
Only increases the rate of reaction
3
New cards
.
4
New cards
.