pchem exam 1
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
50 Terms
which of the following properties is/are related to drug action?
water solubility and steriochemistry
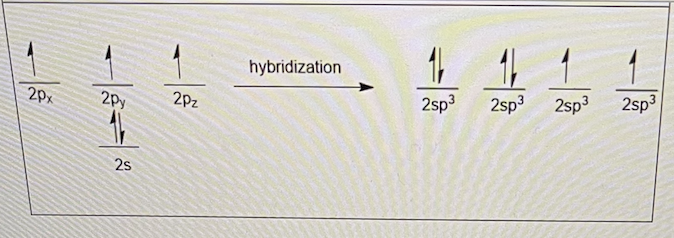
oxygen, a group 6 element, has 6 valence electrons shown on the left. after sp3 hybridization, the valence electrons are distributed as shown on the right - each orbital gets one electron before any orbital gets paired up. this is due to which effect?
hunds rule
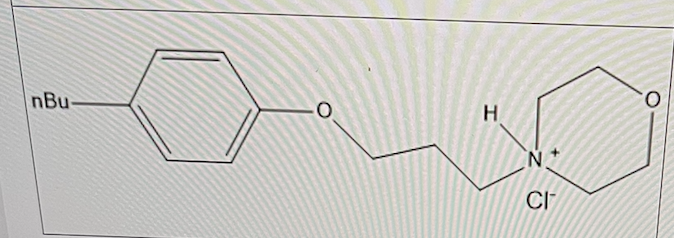
True or False:
The pKa of Pramoxine hydrochloride is 7. The pKb of the amine (free base form) would also be 7.
true
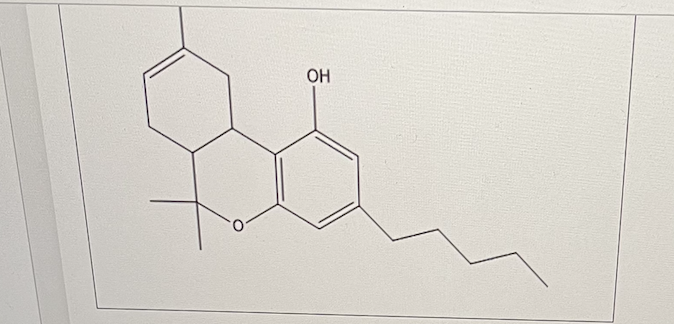
For 11-nor-9-carboxy-9-THC, calculate the percent ionization of the carboxyl center in the duodenum (round up or down to whole number).
91%
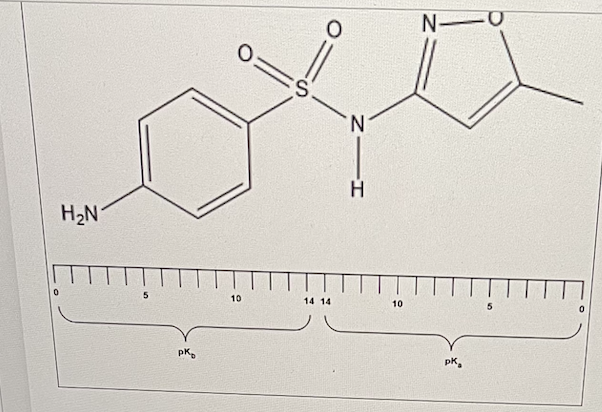
Using the pKa-pKb continuum provided and the A pK tables, predict a pKb value for Sulfamethoxazole, an oral antibiotic.
pKb = 5
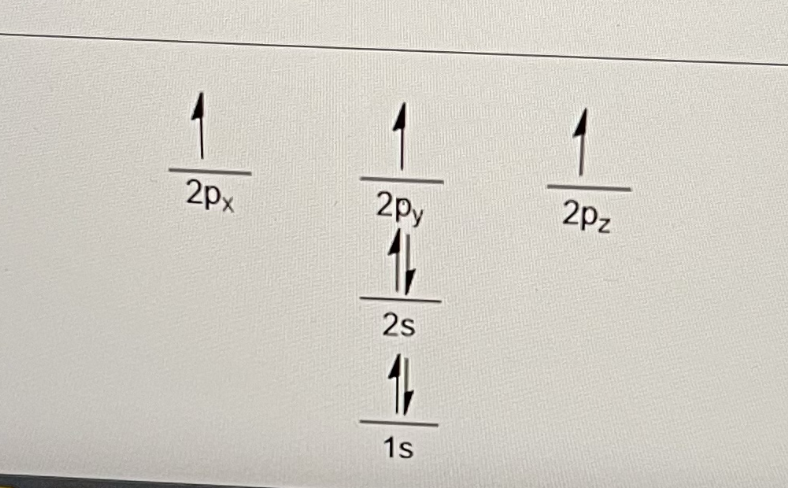
The figure below shows the electronic configuration of nitrogen, a Group V element with 5 valence electrons. The electrons are distributed such that the lower energy orbitals are filled first with 2 electrons before the higher energy orbitals.
This is known as what?
the Aufbau principle
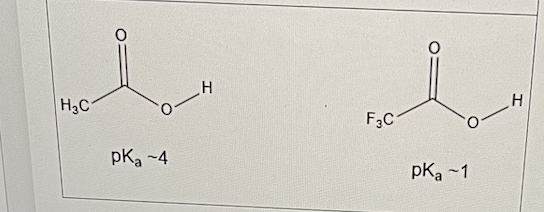
Trifluoroacetic acid is a stronger acid than acetic acid, i.e it can give up its proton much more readily than acetic acid.
This is mainy due to what?
an inductive effect

Examine the molecule shown.
The 5-membered ring would appear to be aromatic at first glance, but it is not. The lack of aromaticity in the 5-membered ring is due to which of the following?
The 5-membered ring does not have the required number of π-electrons
Which of the following is used to prevent tooth decay?
Na2SiF6
True or False:
A conjugated system is lower in energy because electron density can be dispersed over multiple atoms rather than remain localized at only one atom.
true
True or False:
The number of atomic or hybrid orbitals that go into bond formation must equal the number of bonding orbitals that result from bond formation.
true
True or False:
According to the Lewis acid-base theory, a molecule that can accept a pair of electrons is said to be a Lewis acid.
true
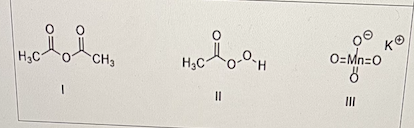
which of the following is/are an oxidizing germicide(s)?
II and III only
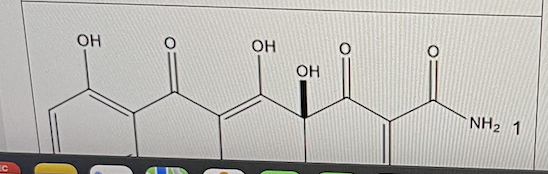
Tetracycline is a commonly used antibiotic.
Using the pKa-pKb continuum and the pK tables, predict a pKa value for the amide nitrogen labeled 1 below.
pKa = 14
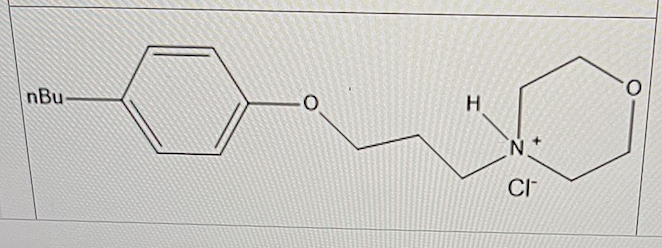
For the analgesic Pramoxine hydrochloride, the pKa was experimentally determined to be 7.
In the blood plasma, what is the % ionization (round up or down to whole numbers)?
50%
Alka-Seltzer is a combination of NaHCO and citric acid plus other ingredients).
What is/are the active species that directly neutralize the acid causing heartburn?
I. NаHCОз
II. Sodium citrate
III. Citric acid
I and II only
The trifluoromethyl group has a strong electron withdrawing effect. A single fluorine, however, shows a much weaker withdrawing effect.
Which of the following statements best explains the decreased electron withdrawing effect of a single fluorine?
R-CH2-CF3
R-CH2-F
A single fluorine can back donate electrons into an adjacent o-antibonding orbital
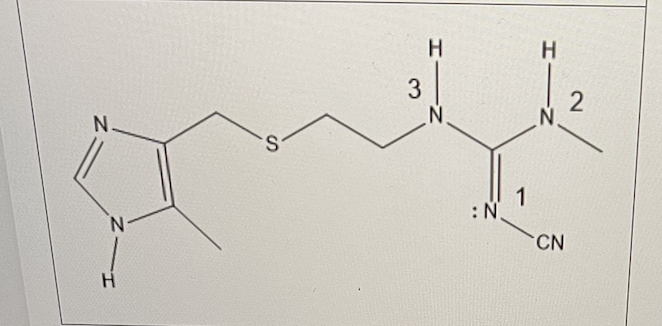
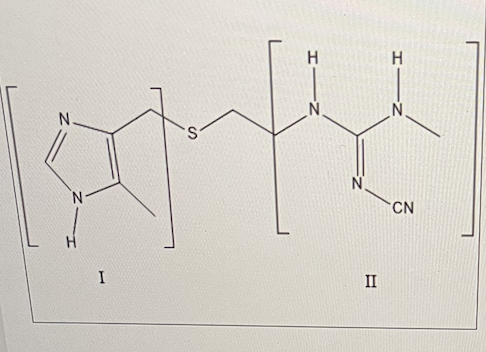
For Cimetidine (Tagamet) shown below, the nitrile group on nitrogen 1 will have what effect on the pKb of that nitrogen?
raise the pKb
Which of the following is/are correct about the phase I clinical trial?
I. It usually involves less than one hundred volunteers.
Il. One purpose is to evaluate the efficacy of a new drug candidate.
III. One purpose is to evaluate the prescribing behavior of physicians.
I only
Which of the following affects) a drug's action after i.v. administration?
I. distribution
II. metabolism
III. elimination
I, II, and III
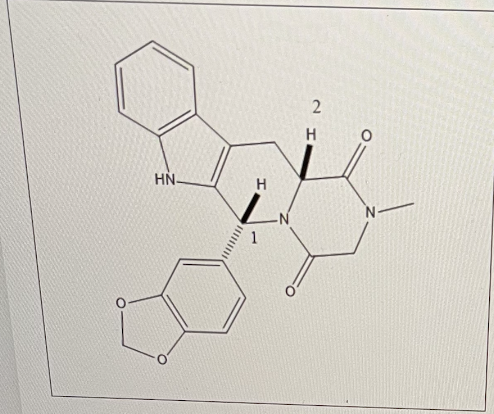
what describes the drug?
neutral
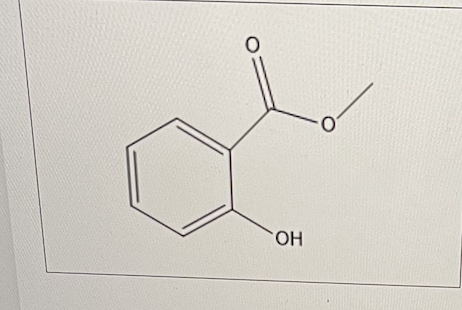
For methyl salicylate (oil of wintergreen), the ester group at the ortho position has what effect on the pKa of the phenol?
lowers the pKa
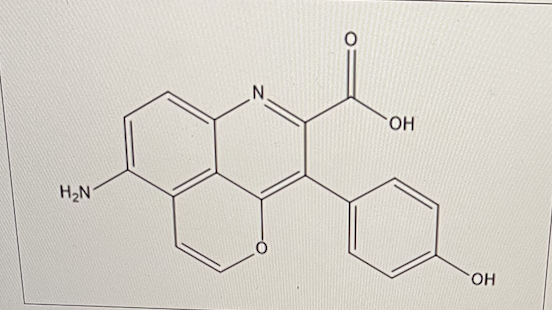
for the molecule given below, how many aromatic rings are there?
3
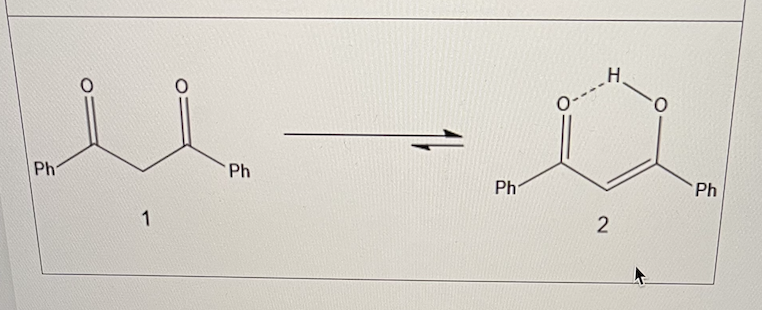
the conversion of 1 to 2 is an example of what?
tautomerism
A value of 12 is estimated for the pKa using the pKa-pKb continuum.
Using the Bronsted-Lowry definitions of acids and bases, what is the molecule?
acidic
The bond angle for a sp2-hybridized carbon is
120 degrees
Which of the following is/are sources of drugs?
I. chemical synthesis
II. microorganisms
III. plants
I, II, and III
Which of the following values in the Lipid Panel is/are experimentally determined?
I. Total cholesterol
II. HDL
III. LDL
I and II only
The purposes) of a phase Il clinical trial is/are
I. to demonstrate efficacy in treating the intended diseases
II. to assess the drug's therapeutic value in special population
Ill. to look for rare side effects
I only
True or False:
The pH is a function of the molecule.
False
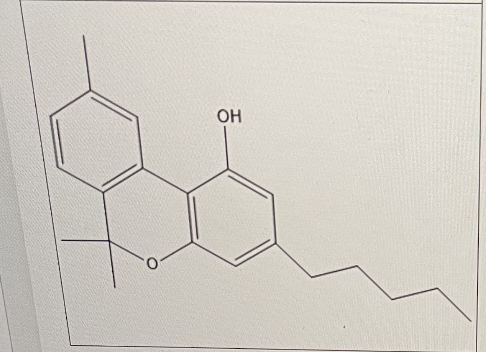
How many aromatic rings are there in cannibinol?
2
Drug-target interactions can involve
I. covalent interactions
Il. hydrophobic interactions
III. ionic interactions
I, II, and III
What is the structure in bracket I?
imidazole
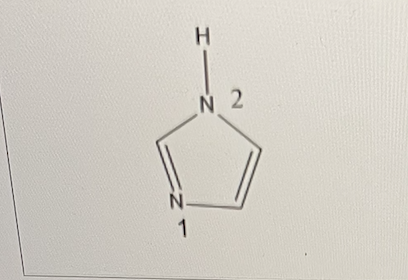
In imidazole, nitrogen 1 is more basic than nitrogen 2.
What is this due to?
A. The lone pair on nitrogen 2 is perpendicular to the -system in the ring.
B. The lone pair on nitrogen 2 is co-planar to the -system in the ring.
C. The lone pair on nitrogen 1 is perpendicular to the -system in the ring.
D. The lone pair on nitrogen 1 is co-planar to the -system in the ring.
B and C
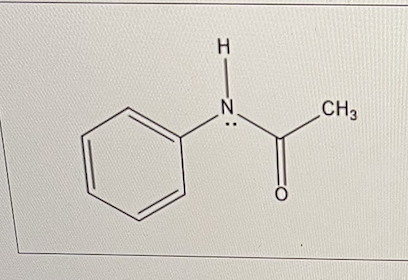
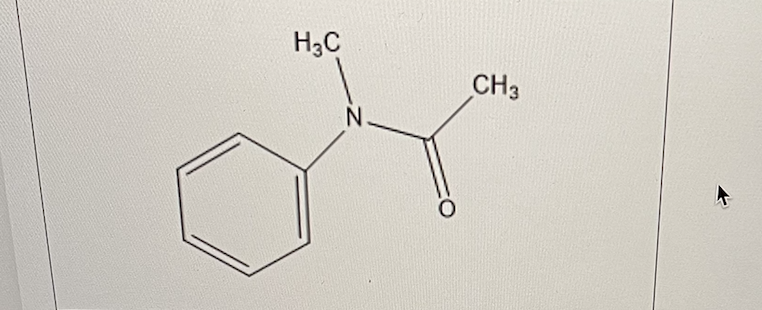
In N-acetylaniline, the amide group has an electron withdrawing effect on the methyl group.
What effect does it have on the aromatic ring?
It has a modest electron donating effect
If a molecule can neither donate a proton (Ht) nor accept it, then by the Bronsted-Lowry acid-base concept, it is said to be what?
a. amphoteric
b. hydrophilic
c. hydrophobic
d. neutral
neutral
The following drug
I. acts as an antacid.
Il. has anti-inflammatory activity.
III. has antibacterial activity.
I, II, and III
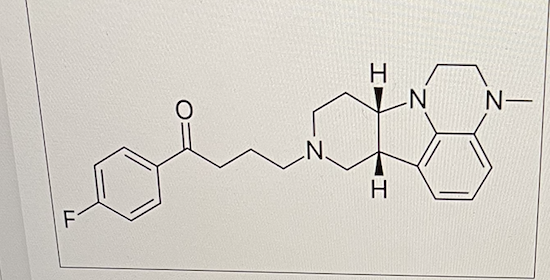
The drug illustrated below is most likely absorbed in __ after oral administration
the intestines only
Which of the following statements about functional groups are true?
I. They affect binding to receptor sites.
II. They affect the drug's stability during storage.
III. They affect a drug's metabolism in the body.
IV. They affect a drug's biological response.
I, II, III, and IV
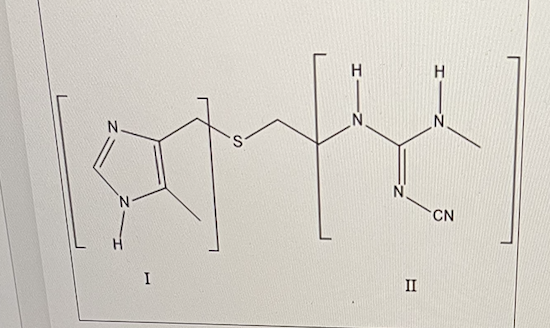
For Cimetidine (Tagamet) shown below, what is the name of the functional group in bracket II? (ignore the CN in this case)
guanidine
lodized salt can prevent
I. goiter
II. mental retardation
Ill. tooth decay
I and II only
Arrange the following oxidizing germicides in the order of oxidizing potential. (from high to low)
I. I2
II. H2O2
III. Hypochlorous acid
II>III>I
Which condition(s) must be met for a molecule to be antiaromatic?
a. The molecule must by cyclic
b. The molecule must have 4n π- electrons
c. Each atom in the ring must contribute a p-orbital
d. The molecule must be flat
e. All of the above
all of the above
Alkyl groups such as methyl, ethyl, propyl, isopropyl etc, can donate electrons to a positively charged center (or one with increased positive charge density) through what effect?
a. aromatization
b. back donation
c. hyperconjugation
d. induction
hyperconjugation
True or False:
Orbitals that are perpendicular to each other can form covalent bonds.
False
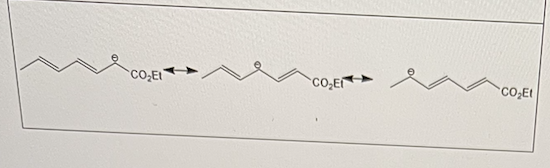
In the diagram below, the negative charge adjacent to the ester group can be "walked" down the chain.
This effect is known as what?
delocalization
Which of the following is/are correct about the drug discovery and development process?
I. Of each 10,000 compounds synthesized, only about 5 will likely be tested in human clinical trials.
II. It usually takes about 12-15 years to develop a new drug and put it in the market.
IlI. On average, it costs around $100,000,000 to discover a new drug.
I and II only
Most drugs
I. are organic molecules.
Il. require a specific biological target to produce their biological activity.
III. have more water solubility than lipid solubility.
I and II only
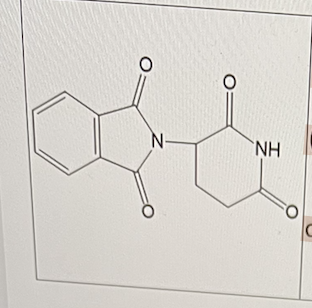
Which of the following is/are correct about thalidomide?
I. It was withdrawn in early 1960s due to severe teratogenic side effects (causing birth defect).
II. It is still currently used clinically.
Ill. It contains 2 chiral centers.
I and II only
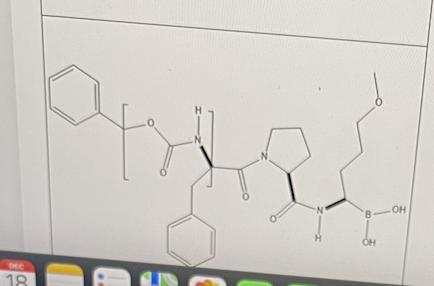
Flovagatran is used to treat thrombosis.
Identify the functional group in the bracket.
carbamate