VSEPR + Hybridization
Electron Groups | Bonding Groups | Lone Pairs | Electron Geometry | Molecular Geometry | Approximate Bond Angles | Hybridization | Example |
2 | 2 | 0 | Linear | Linear | 180° | sp |  |
3 | 3 | 0 | Trigonal planar | Trigonal planar | 120° | sp2 | 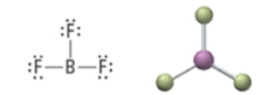 |
3 | 2 | 1 | Trigonal planar | Bent | <120° | sp2 |  |
4 | 4 | 0 | Tetrahedral | Tetrahedral | 109.5° | sp3 | 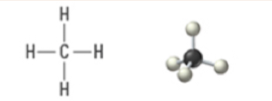 |
4 | 3 | 1 | Tetrahedral | Trigonal pyramidal | <109.5° | sp3 |  |
4 | 2 | 2 | Tetrahedral | Bent | <109.5° | sp3 |  |
5 | 5 | 0 | Trigonal bipyramidal | Trigonal bipyramidal | 120° (equatorial) 90° (axial) | sp3d | 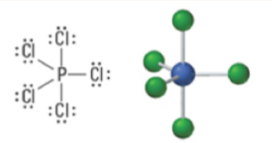 |
5 | 4 | 1 | Trigonal bipyramidal | Seesaw | <120° (equatorial) <90° (axial) | sp3d | 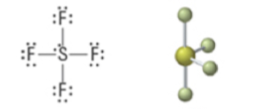 |
5 | 3 | 2 | Trigonal bipyramidal | T-shaped | <90° | sp3d | 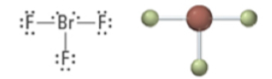 |
5 | 2 | 3 | Trigonal bipyramidal | Linear | 180° | sp3d |  |
6 | 6 | 0 | Octahedral | Octahedral | 90° | sp3d2 | 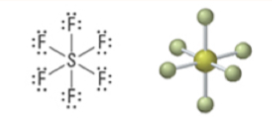 |
6 | 5 | 1 | Octahedral | Square pyramidal | <90° | sp3d2 | 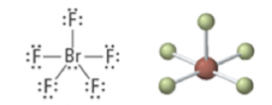 |
6 | 4 | 2 | Octahedral | Square planar | 90° | sp3d2 | 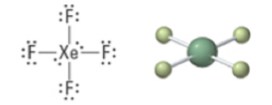 |