AP Chemistry - Chapter 8
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
57 Terms
(8.1) What is a chemical bond?
A chemical bond is when a chemical reaction occurs between two atoms, and some of their electrons are reorganized, resulting in a net attractive force between the atoms.
(8.1) What is ionic bonding?
It's the attraction between a positive ion and a negative ion resulting from the complete (or nearly complete) transfer of one or more electrons from one atom to another.
(8.1) What is covalent bonding?
It's an interatomic attraction resulting from the sharing of electrons between the atoms.
(8.1) What is metallic bonding?
It's the atoms in an extended lattice are immersed in a sea of delocalized electrons
(8.1) What are core electrons and valence electrons? How are each involved in bonding?
Valence electrons are the s and p electrons in the outermost shell.
Core electrons are the electrons that are in the inner shells of the electrons.
Valence electrons can be lost, gained, or rearrangement in chemical reactions while core electrons are not involved.
(8.1) What are the Lewis dot symbols for the Period 2 elements?
The Lewis dot symbols for Period 2 elements have 2 dots.

(8.1) What rule must Lewis structures obey when drawing molecules? Why?
They must obey the octet rule in order to achieve a stable configuration of having eight valence electrons.
(8.2) What are the symbols for the following:
lone pair
bonding pair
double bond
triple bond

(8.2) In general, which atom should be placed in the center of a Lewis dot structure?
In general, the carbon atom is often placed in the center of a Lewis dot structure. If not carbon then the least electronegative atom will be in the center.
(8.2) What do NO+, N2, CO, and CN- have in common? What do they call these related species?
They are similar because they each have two atoms and the same total number of valence electrons which leads to a similar Lewis structure for each molecule or ion.
They call these species isoelectronic species.
(8.1-8.2) What are the octet rule exceptions?
Beryllium (Be) can be stable with only two bonds.
Boron (B) can be stable with only three bonds
3rd row and heavier elements CAN exceed the octet rule using empty valence d orbitals
(8.1-8.2) HONC Rule
Hydrogen and Halogen: form one covalent bond
Oxygen (+Sulfur): form two covalent bonds
Nitrogen (+Phosphorus): form three covalent bonds
Carbon (+Silicon): form four covalent bonds
(8.4) What are resonance structures?
They are the possible structures of a molecule for which more than one Lewis structure can be written.
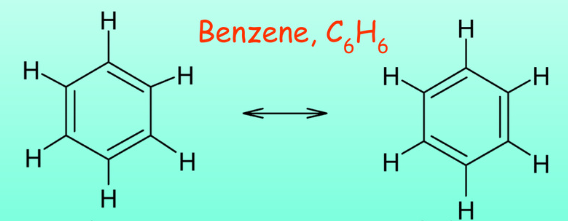
(8.4) TRUE OR FALSE: The resonance structures of the structure shown are accurate to real life.
FALSE: The actual structure is the average of the resonance structures.
Despite the use of single and double bonds in the model, the actual bond lengths in the ring are identical (the average of single and double bonds)
(8.4) What is the length and strength of resonance bonds compared to the length and strength of single bonds and double bonds?
Resonance bonds are shorter and stronger than single bonds, but longer and weaker than double bonds.
(8.4) What are nonequivalent resonance structures?
It’s when the resonance structures have different numbers of single and double bonds. This means that one resonance structure is more stable than another. In this case you need to calculate the formal charge of the structures to see which form is most likely to exist.
(8.3) How to determine the formal charge of a structure:
Formal Charge on an Atom = (# of valence e- in the free neutral atom) - (# of valence e- assigned to the atom in the molecule)
Count ½ the electrons in each bond as “belonging“ to the atom.
Loan pairs “belong“ entirely to the atom they are attached to.
(8.3) How to know which resonance structure is most likely to exist:
Atoms in molecules should have formal charges as close to zero as possible
Any negative formal charges should be on electronegative atom
The sum of the formal charges of all atoms in a given molecule or ion must equal the overall charge on that species
(8.5) What is a coordinate covalent bond? How are they symbolized in Lewis structures?
They are interatomic attractions resulting from the sharing of a lone pair of electrons from one atom with another atom. They are symbolized by an arrow that points away from the atom donating the electron pair.
(8.5) What does hypervalent mean?
It means a compound in which the central atom is surrounded by more than four valence electron pairs.
(8.5) What is a free radical?
It is a chemical species containing an unpaired electron.
(8.6) What is VESPR theory?
It is a model for predicting the shapes of molecules, in which structural electron pairs are arranged around each atom to maximize the angles between them.
(8.6) Electron Domains
bonded or lone pairs of electrons about a central atom
lone pairs as well as single, double, and triple bonds all behave individually as a SINGLE domain
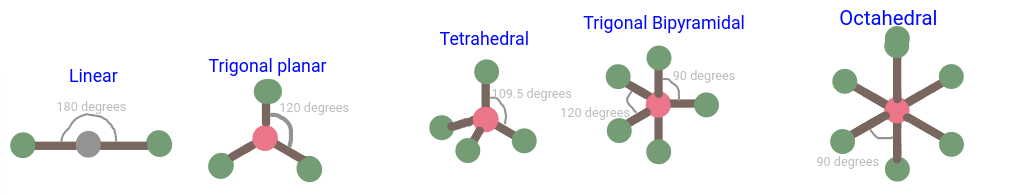
(8.6) Sketch and label the 5 geometric shapes predicted by VSEPR – include their angles.
(8.6) Electron Pair Geometry vs Molecular Geometry
Electron Pair Geometry: the shape of the molecule due to the arrangement of atoms — including lone pairs.
Molecular Geometry: the shape of the molecule due to the arrangement of atoms — excluding lone pairs.
(8.6) List the Molecular Geometry and Include:
name of molecular geometry
# of domains
# of unpaired electrons
bond angles
Linear - 2 domains - 0 unpaired electrons - 180 degrees
Trigonal Planar - 3 domains - 0 unpaired electrons - 120 degrees
Bent - 3 domains - 1 unpaired electron - <120 degrees
Tetrahedral - 4 domains - 0 unpaired electrons - 109.5 degrees
Trigonal Pyramidal - 4 domains - 1 unpaired electrons - <109.5 degrees
Bent - 4 domains - 2 unpaired electrons - <109.5 degrees (water is 104.5 degrees)
Trigonal Bipyramidal - 5 domains - 0 unpaired electrons - 90 and 120 degrees
See-Saw - 5 domains - 1 unpaired electrons - <90 and 120 degrees
T-Shaped - 5 domains - 2 unpaired electrons - <90 and 120 degrees
Linear - 5 domains - 3 unpaired electrons - <90 and 120 degrees
Octahedral - 6 domains - 0 unpaired electrons - 90 degrees
Square Pyramidal - 6 domains - 1 unpaired electrons - <90 degrees
Square Planar - 6 domains - 2 unpaired electrons - <90 degrees
(8.6) How do you know if a molecule is polar based on its Lewis Structure?
Any molecule of just two elements with “significantly“ different electronegativities is polar
Any molecule whose dipoles do not cancel will be polar
Look for asymmetry and/or more than one kind of atom attached to the central atom
(8.6) What is the effect of lone pairs on bond angles?
The effect that lone pairs have on bond angles is that it causes the bond pairs to squeeze closer together.
(8.7) What is a polar covalent bond?
It is a covalent bond in which there is unequal sharing of the bonding electron pair.
(8.7) What causes the displacement of bonded electrons toward a particular atom?
The displacement of bonded electrons toward a particular atom happens if it has a larger share of the electron pair and has a partial negative charge. The atom at the other end of the bond is depleted in electrons and has a partial positive charge.
(8.7) How are charges represented in ionic bonds versus polar covalent bonds?
In ionic compounds, charges are represented with + and - symbols and are written alongside the atom symbols in the Lewis drawings. In polar covalent bonds, the symbols are δ+ and δ-.
(8.7) How is electronegativity defined?
It measures the relative tendency for an atom to polarize a bond and the ability of an atom in a molecule to attract electrons to itself.
(8.7) What is the relationship between electronegativity and the ionic character of the bond?
As electronegativity increases, so does the ionic character of the bond.
(8.7) What is the general range of electronegativity values for metals? metalloids? nonmetals?
Metals: less than 1 to around 2
Metalloids: around 2
Nonmetals: more than 2
(8.7) What is charge distribution?
It is the way electrons are distributed in a molecule or ion.
(8.7) What are the two guidelines to use when describing charge distributions in molecules and ions?
The first guideline used is the principle of electroneutrality in which electrons are distributed in such a way that the charges on all atoms are as close to zero as possible. The second guideline is that if a negative charge is present, it should be from the most electronegative atoms.
(8.7) What determines the overall polarity of a molecule?
The polarity of the individual bonds in a molecule.
(8.7) homonuclear diatomic molecule vs heteronuclear diatomic molecule
homonuclear: molecules containing 2 atoms of the same element
heteronuclear: molecules containing 2 atoms of the different elements
(8.7) TRUE OR FALSE: Homonuclear diatomic molecules are nonpolar.
TRUE
(8.7) In the molecule HF, which part of the molecule will have more electron density? Why?
The Fluorine(F) will have more electron density because it has a higher electronegativity than Hydrogen(H). This means the electrons in the bonds are skewed towards the F atom and the shared electrons shift towards the F atom.
(8.7) What is the relationship between electronegativity and polarity?
The greater the difference in electronegativity, the more polar the covalent bond.
(8.7) Rank the bonds from greatest polarity to least: H vs. F | C vs. F | O vs. F
H—F > C—F > O—F
(8.7) Which has the greatest polarity:
N—O; K—F; B—N; S—Cl
Tip: Which elements are farthest from each other on the table.
K—F
(8.8) How is molecular polarity measured?
It is measured by seeing if the electron density is gathered more towards one side of the molecule.
(8.8) TRUE OR FALSE: Most molecules have polar bonds.
TRUE: Even if the overall polarity of the molecule is nonpolar, the individual bonds in the molecule are polar.
The only exception is if there is a bond in which 2 of the elements are the same.
(8.8) What does molecular polarity depend on?
Molecular geometry, symmetry, and charge distribution.
If centers of + and - charges ARE NOT the same, this means that the molecule possesses a not dipole moment and IS POLAR
Whenever there exists a line or plane of asymmetry, the molecule IS POLAR
(8.9) POLAR covalent bonds vs NONPOLAR covalent bonds
POLAR: electrons are unequally shared - EN difference of 0.5 to 1.7
NONPOLAR: electrons are equally shared - EN difference of 0.0 to 0.5
(8.9) 3 Factors that Bond Length and Energy Depend On:
electron-electron repulsive forces
proton-proton repulsive forces
electron-proton attractive forces
(8.9) What determines the optimal bond length?
Lowest energy point of the two atoms
STUDY THIS DIAGRAM (its on the AP test)

(8.9) What is bond length and how is it related to the sizes of the atoms?
It is the distance between the nuclei of two bonded atoms.
If an atom is bigger its bond length would be bigger due to the atom's electron cloud taking up more space.
(8.9) What is bond order? How is it calculated?
It is the number of bonding electron pairs shared by two atoms in a molecule.
Bond order is calculated by using the equation:
Bond order = (total # of e- pairs used for a type of bond)/(total # of bonds of that type)
(8.9) Fractional Bond Order
Occur in molecules with resonance structures.
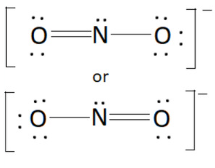
(8.9) Find the Bond Order of NO2-
Bond order = (total # of e- pairs used for a type of bond)/(total # of bonds of that type)
Bond order = (3 e- pairs used in N—O bond)/(2 N—O bonds)
The N—O bond order = 1.5
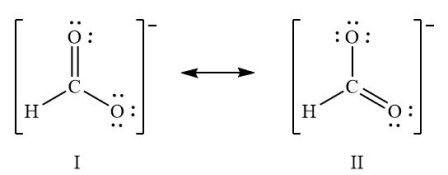
(8.9) What is the average carbon-oxygen bond order of the formate ion, HCO2-?
Bond Order = (# of bonds)/(# of domains)
Bond Order = (3 bonds)/(2 domains)
Bond Order = 1.5
(8.9) Bond Properties:
Order
Length
Energy (Strength)
Order: Bond order may be 1, 2 and 3 as well as fractional values
Length: Bond length is inversely proportional to bond order (Decreased Bond Length = Increased Bond Order)
ex) O=O is shorter than O—O bond
Energy (Strength):
Bond strength increases with bond order
ex) O=O is stronger than O—O bond
Bond strength also increases with the difference in electronegativity between two covalently bonded atoms
ex) HCl bond is stronger than the HBr bond
(8.9) Bond (Dissociation )Energy Equation
ΔH = Energy Required (Reactants) - Energy Released (Products)
(8.9) Calculate Bond Dissociation Enthalpy of the Equation:
1/2N2(g) + 3/2F2(g) → NF3(g)
N≡N: 946 | F-F: 159 | N-F: 272
ΔH = Energy Required (Reactants) - Energy Released (Products)
ΔH = ( 1/2(946) + 3/2(159) ) - ( 3(272) ) = -105kJ/mol