CHEM 120 Chapter 13
1/58
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
59 Terms
Mixtures
2+ components
-Homogenous/heterogenous
Homogenous mixtures
Solutions
Have a uniform appearance
Can have all 3 states as a base
Can have multiple states in one solution
Water base = “aqueous”
A clear solution is ___ not ____
see through, not colorless
heterogenous mixture
can tell the different components apart
ICLICKER
Which of the following is NOT true for solutions?
A) Solutions have a uniform appearance
B) Solutions are a mixture of two or more substances
C) The substance in largest mole amount is the solvent.
D) A mixture is only a solution if the solvent is water.
E) The substance(s) in lesser mole amount is/are the solute(s).
D.
Solvent
does the dissolving
solute
is the dissolved
Solubility
amount of solute that can dissolve per amount of solvent at a given
temperature.
Units: g/L, M, % mass, mass/volume etc.
Like dissolves like
Solute/solvent particle interactions create INTERMolecular forces
Intermolecular forces
Ion-dipole (ion-polar)
Dipole-dipole
Hydrogen bond (H - N,O,F)
Ion-induced dipole (ion-nonpolar)
Dipole-induced dipole (polar-nonpolar)
Dispersion (nonpolar-nonpolar)
Water and solution formation/Intermolecular forces
Water is a dipole.
The dipole-dipole attraction/ H bonds between water molecules
Ion-dipole between ions and water
Creates Hydration shells/ spheres (water molecules that surround individual ions)
ion-ion between ions
Lattice enthalpy
ion-ion attraction energy
-Decreases with larger atom size
-increases with larger charge
Enthalpy of hydration (water)/solvation enthalpy (not water)
ion-dipole attraction energy
ICLICKER
B.
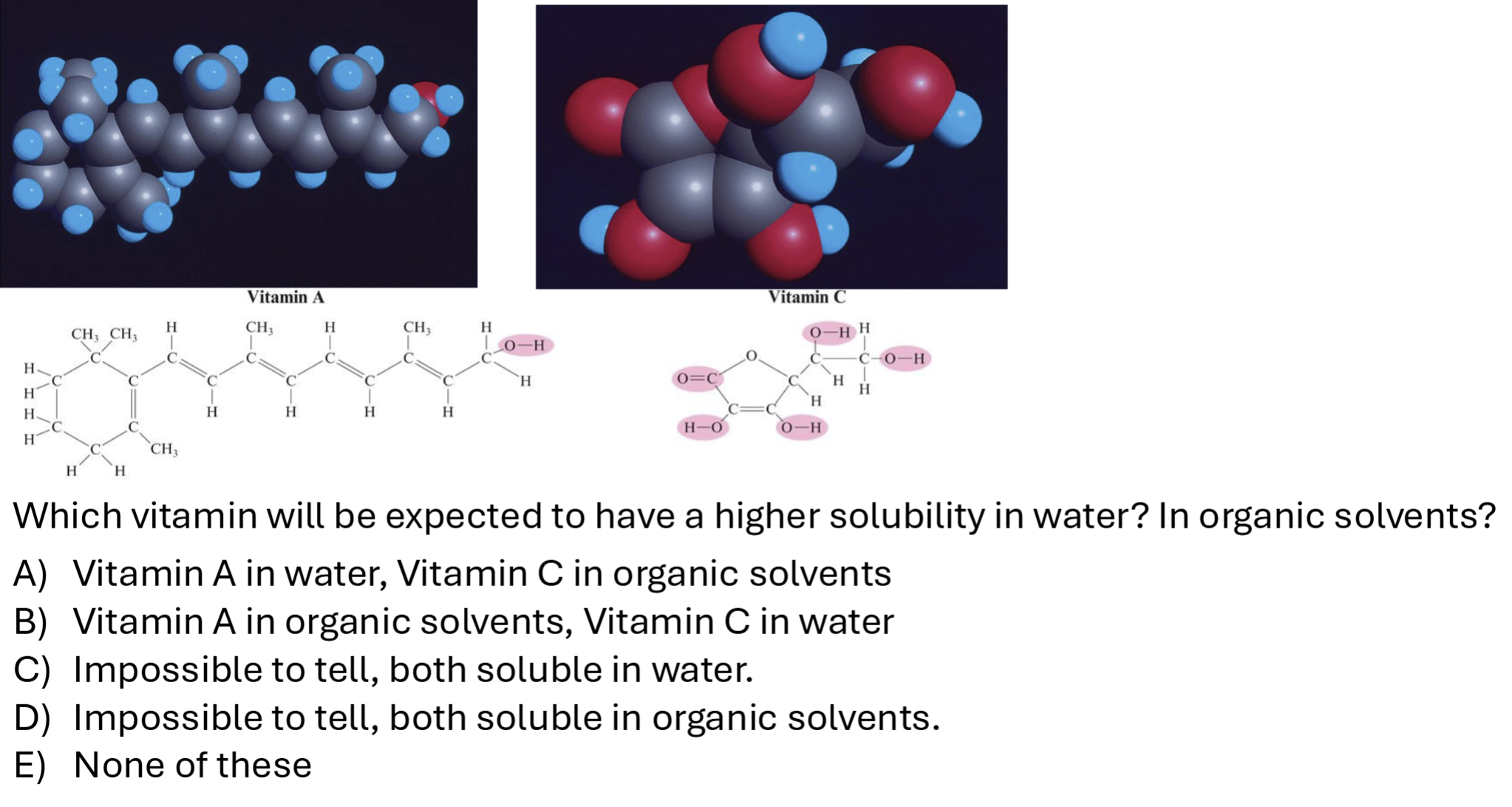
“organic” = fats, etc. nonpolar
nonpolar tail on Vitamin A
multiple polar groups (OH) and dipole (O=C) on vitamin C)
Dual polarity
molecules can have polar and nonpolar ends
form micelles (Polar ends out, nonpolar ends in) which can take in nonpolar molecules into liquid state
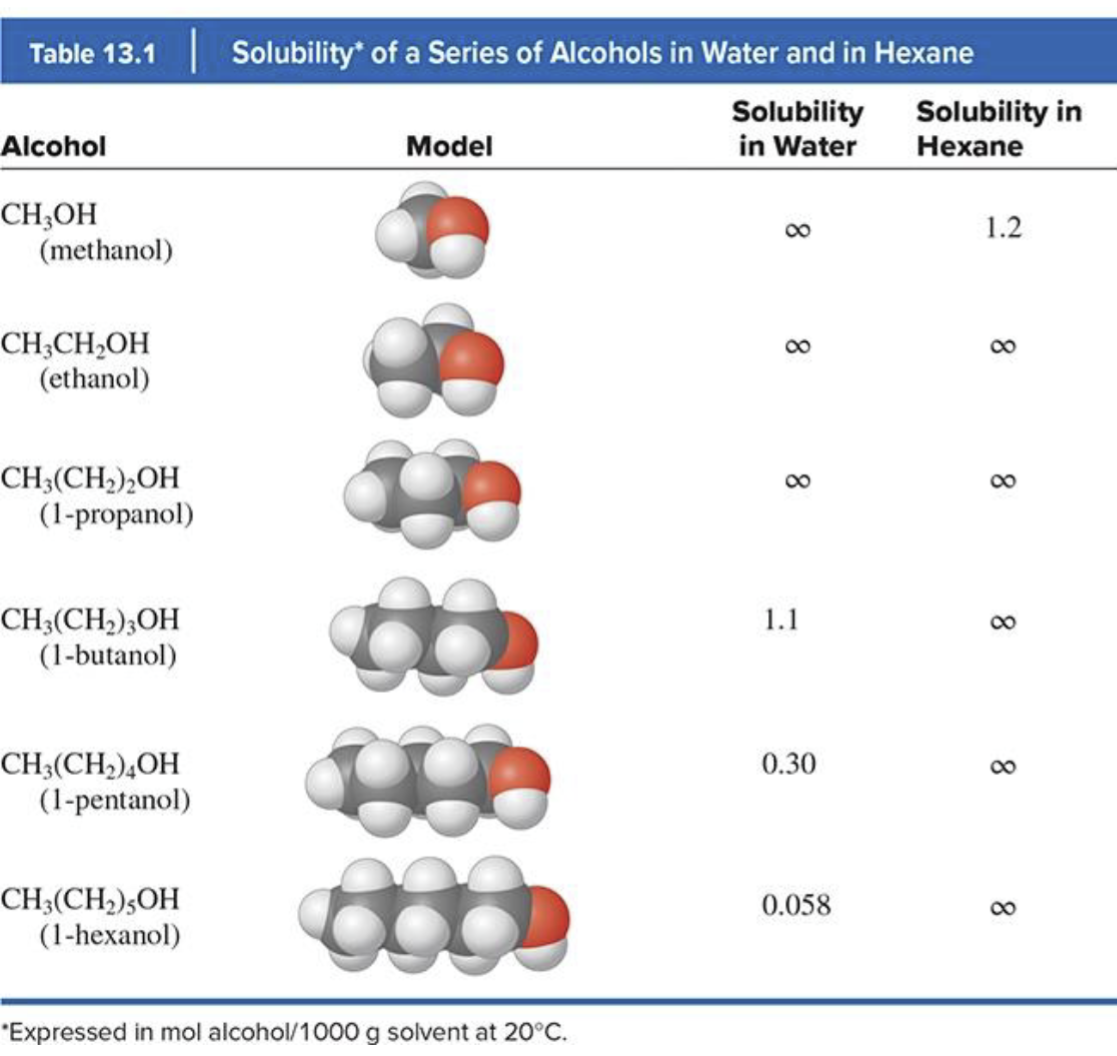
miscibility
ability for a solute to dissolve in all proportions with the solvent
solubility in alcohols in water and in hexane
ICLICKER
Which of the following paired liquids are immiscible in each other?
A) CH3CH2OH and H2O (ethanol and water)
B) NH3 and CH3CH2OH (ammonia and ethanol)
C) CH3CH2CH2CH2CH2CH3 and CH3CH2OH. (hexane and ethanol)
D) CH3CH2CH2CH2CH2CH3 andCH3CH2CH2CH2CH2CH2CH2CH3 (hexane and octane)
E) CH3CH2(OH)CH3 and H2O (isopropanol and water)
None, all are miscible
enthalpy
heat flow at constant pressure (state function- start and end)
denoted by delta H
only denoted by q if not at constant pressure
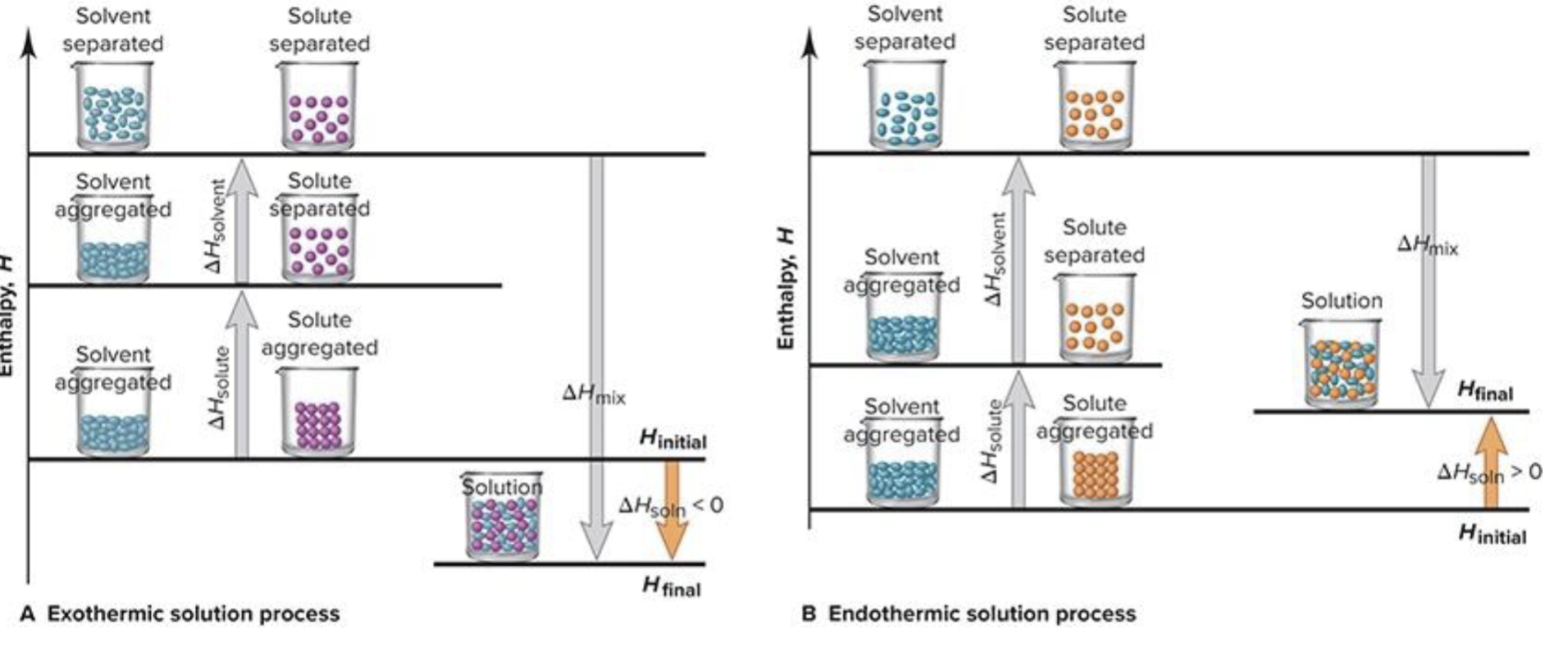
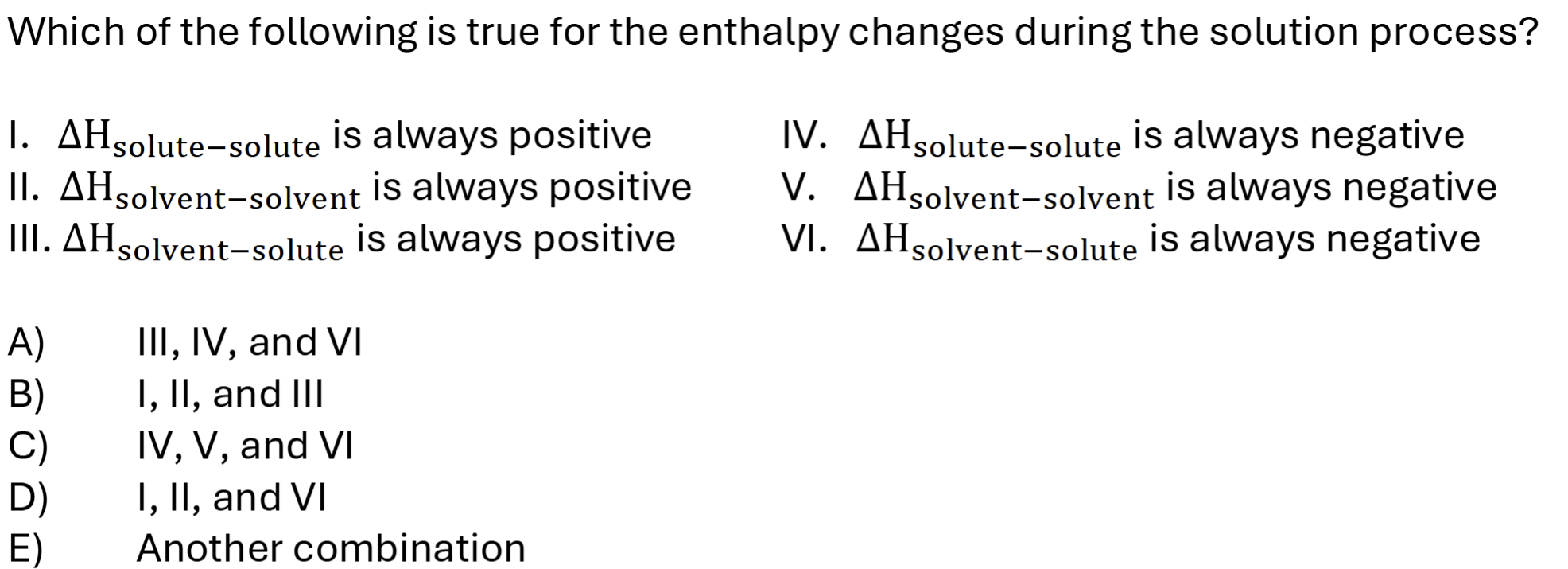
thermodynamics of solution formation
Sum of enthalpy changes
Delta H is positive (colder as it needs more energy to absorb) (total energy of solute and solvent is positive)
If dissolving increases, delta H is more negative due to energy release (delta H mix is always less than 0)
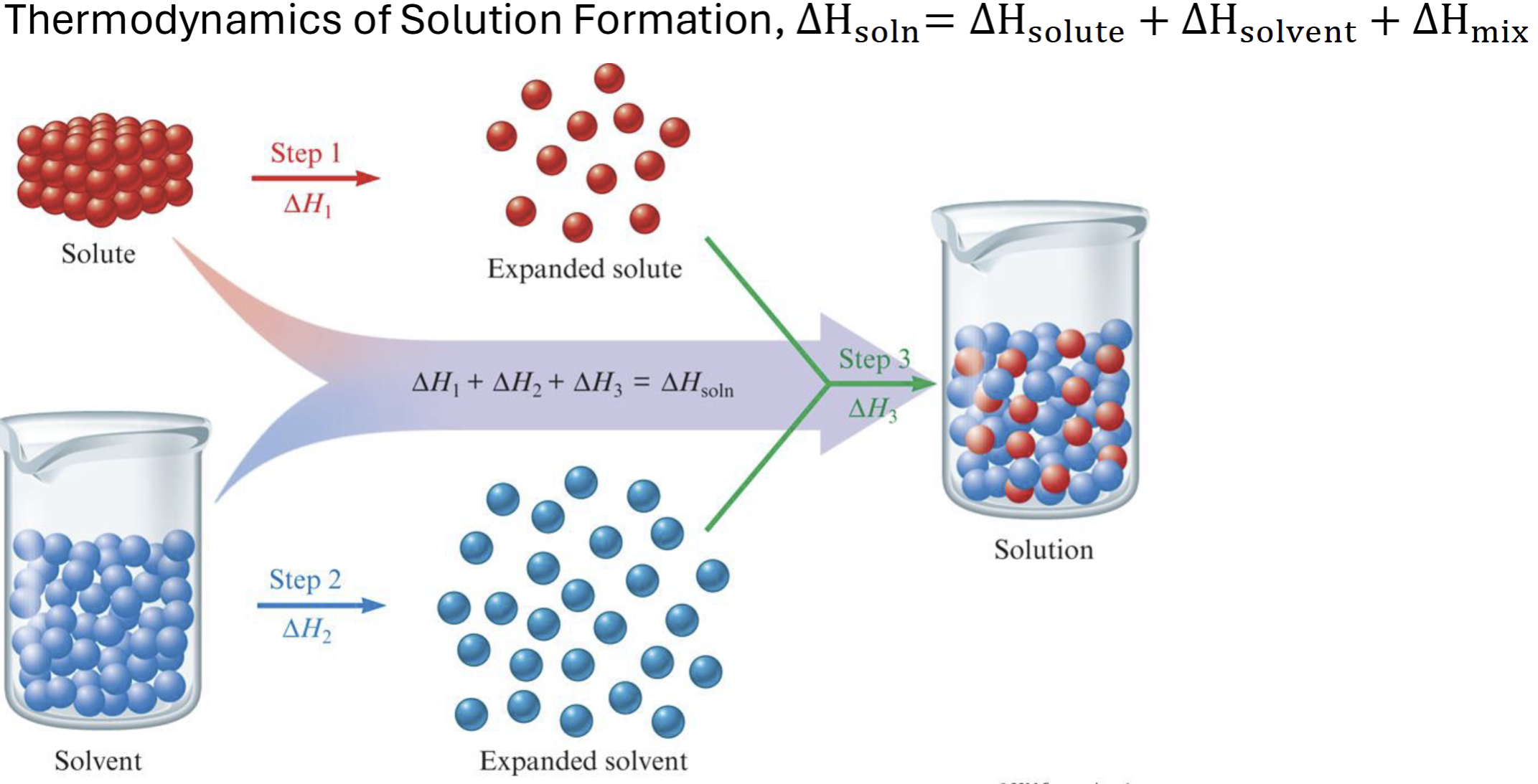
How do you know a solution formation is exothermic or endothermic
H final < H initial - exothermic and Hsolution < 0
H final > initial- endothermic and Hsolution> 0
ICLICKER
D.
Delta H (lattice energy) is >/< 0, delta H (hydration of ions) >/< 0
Lattice energy enthalpy is always positive, hydration of ions are always negative
Ion radius relationship with lattice energy and hydration of ions
The larger the size, heats of hydration and lattice energies increase
Entropy
S
amount of energy dispersed at constant temp.
aka disorder or randomness of a system
Absolute entropies are always positive
0 entropy is at 0 Kelvin
order of phases from least to most entropy
solid
liquid
gas
Dissolving and entropy
dissolving FAVORS entropy as it increases it for example, KCl(s) → KCl(aq) is a very cold liquid and increases the number of particles
equilibrium
a steady state
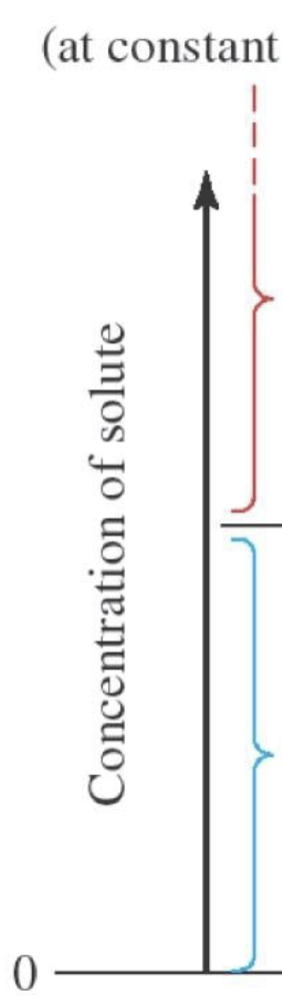
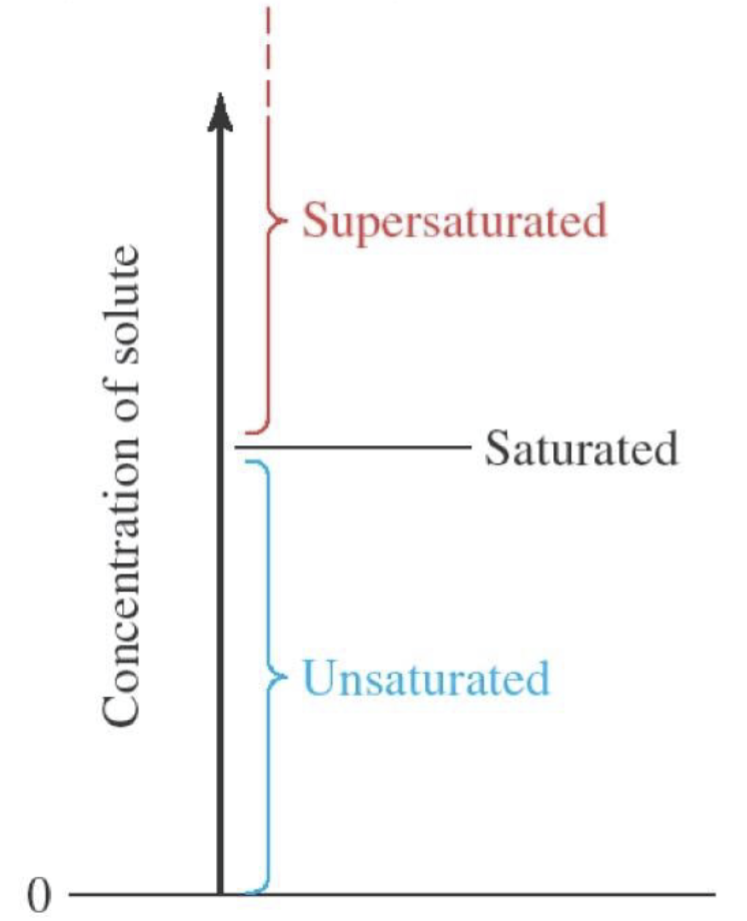
unsaturated solution
solute is dissolved but not at max amount dissolved. there is no solute leftover
saturated solution
max amount of solute dissolved, minimal solute leftover, solution at equilibrium
unit: gSolute/100gSolvent
supersaturated solution
equilibrium releases excess dissolved solute (ex: opening a can of soda releases excess dissolved CO2)
How does temperature affect solid/liquid/gas solutes
As temperature increases, solubility of a solid and liquid solute increases but of a gas solute decreases
As pressure increases, the solubility of a gas in water _
increases (more collisions)
Henry’s Law
Henry’s Law
Sg= kH x Pgas
kH - Henry’s constant (specific to a gas) (the higher kH is, the higher the solubility)
Pgas - Partial pressure of gas

ICLICKER
B. Remember: as temperature increases, the solubility of a gas solute decreases

PRACTICE
Before: 0.17 M CO2
After: 1.36 × 10^-5 M
Molarity
M
concentration at a specific temperature
(solute) mol/ (SOLUTION volume) L
molality
m OR m
concentration independent of temp
(solute) mol / (SOLVENT mass) kg
parts by mass
mass of solute/mass of solutionp
parts by volume
volume of solute/volume of solution
mole fraction
X (percentage before x 100)
(solute) mol / ((solute) mol + (solvent) mol)

PRACTICE
molality- 8.388 m
mole fraction- 0.1313
% by mass- 32.89%

PRACTICE
1) 0.5 m
2) 0.01 m
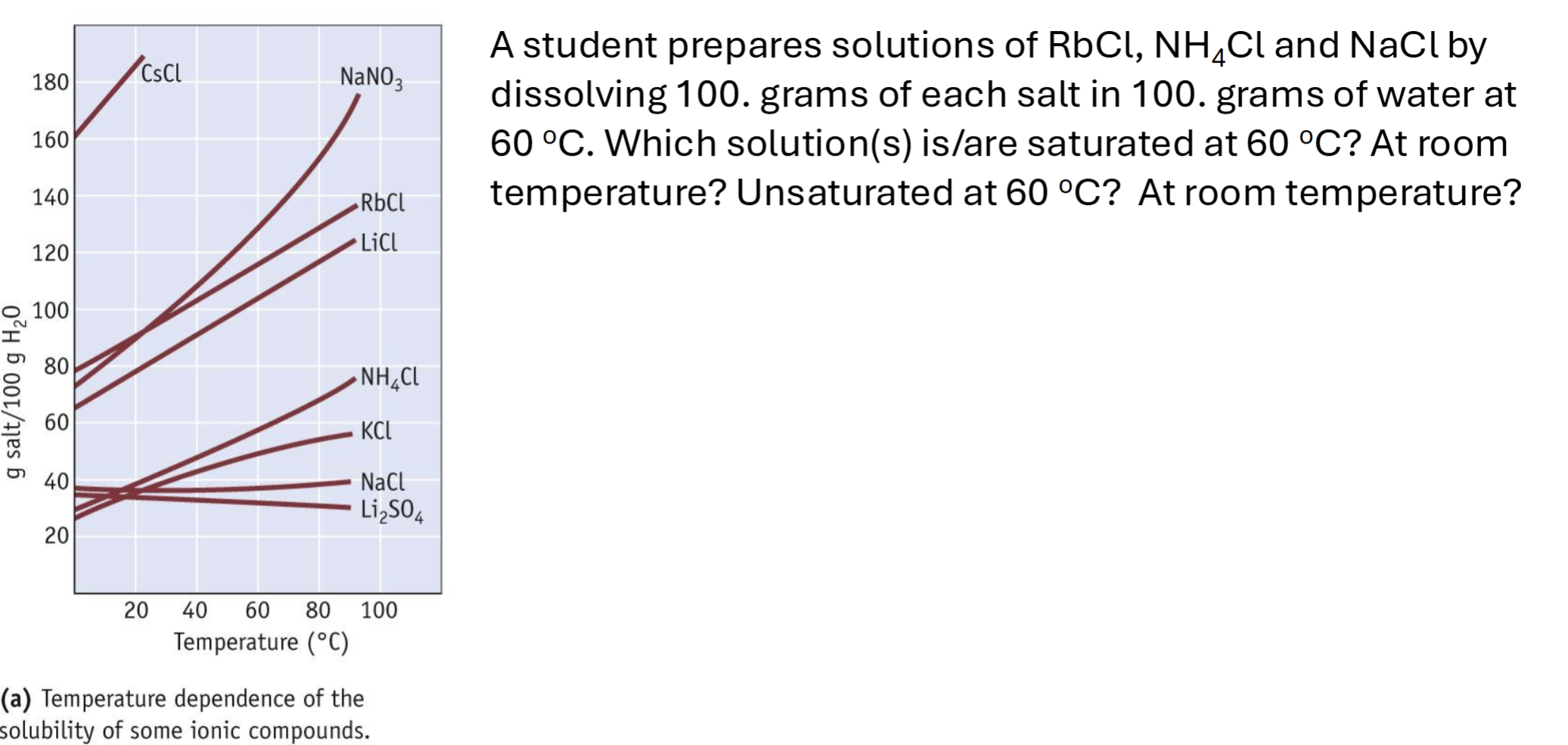
PRACTICE
At 60 degrees: NH4Cl and NaCl are saturated and RbCl is unsaturated
At room temp: all are saturated

ICLICKER
A. 35 is less than 50
Colligative properties
changes in solution properties that depend on the number of solute particles, not the identity of the solute
boiling point elevation, freezing point depression, osmotic pressure, vapor pressure lowering
Dissolving increases boiling point of the solution
electrolytes
solutes that carry a charge through a solution
strong electrolytes
strong acids/bases, soluble ionic compounds
are completely ionized and dissociated
weak electrolytes
weak acids/bases, partially ionized and dissociated
non-electrolytes
sugar, ethylene glycol, ethanol, etc. don’t dissociate or ionize in water
dissociate
forming cations/anions
strong: 2
weak: 1-2
non: 1
Raoult’s Law
property of non-electrolytes
Vapor pressure lowering
VP of a solvent is proportional to mol fraction (as % of the solvent increases, then the final VP is proportional to it
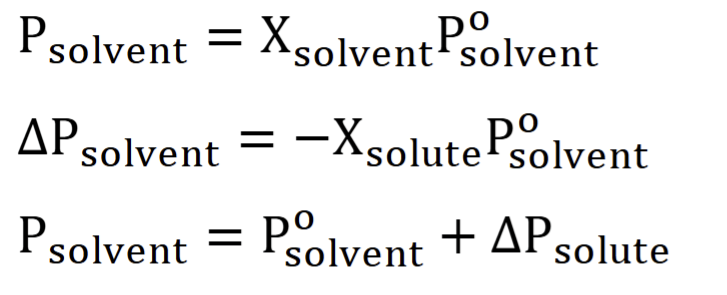
volatile
ability to evaporate
why do nonvolatile solutes lower VP
decreases surface area for evaporation

PRACTICE
23.3 torr
boiling point elevation
Vapor pressure = environmental pressure
solutions always boil at a temp higher than the pure solvent (as molality increases, so does the change in vapor pressure)
1 atm pressure = 100 degrees Celsius (normal boil point)
to compensate, the solution must be heated to a higher temp
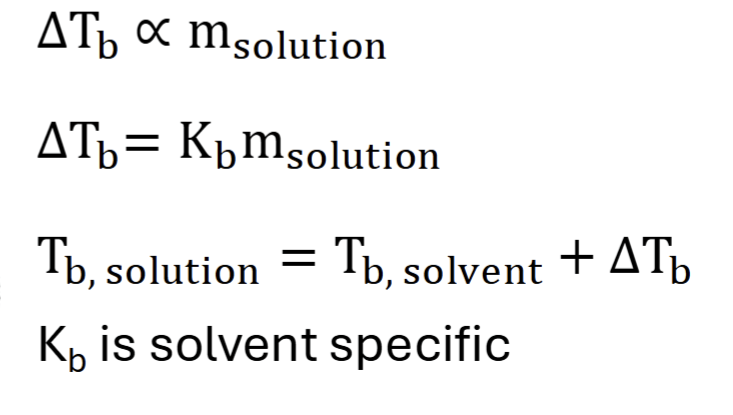
freezing point depression
solutions always freeze at a temp lower than the pure solvent
formation of a lattice by pushing out dissolved solutes
solute disrupts freezing process, solution must be cooled to a lower temp to freeze solvent
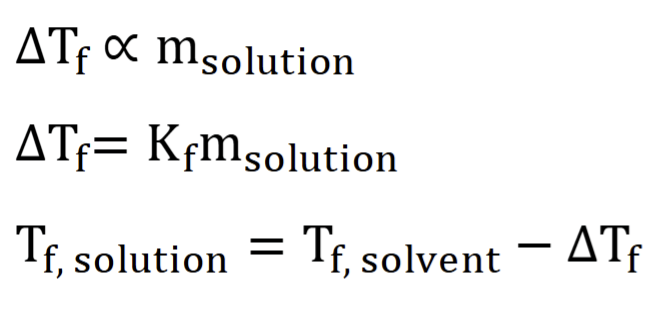
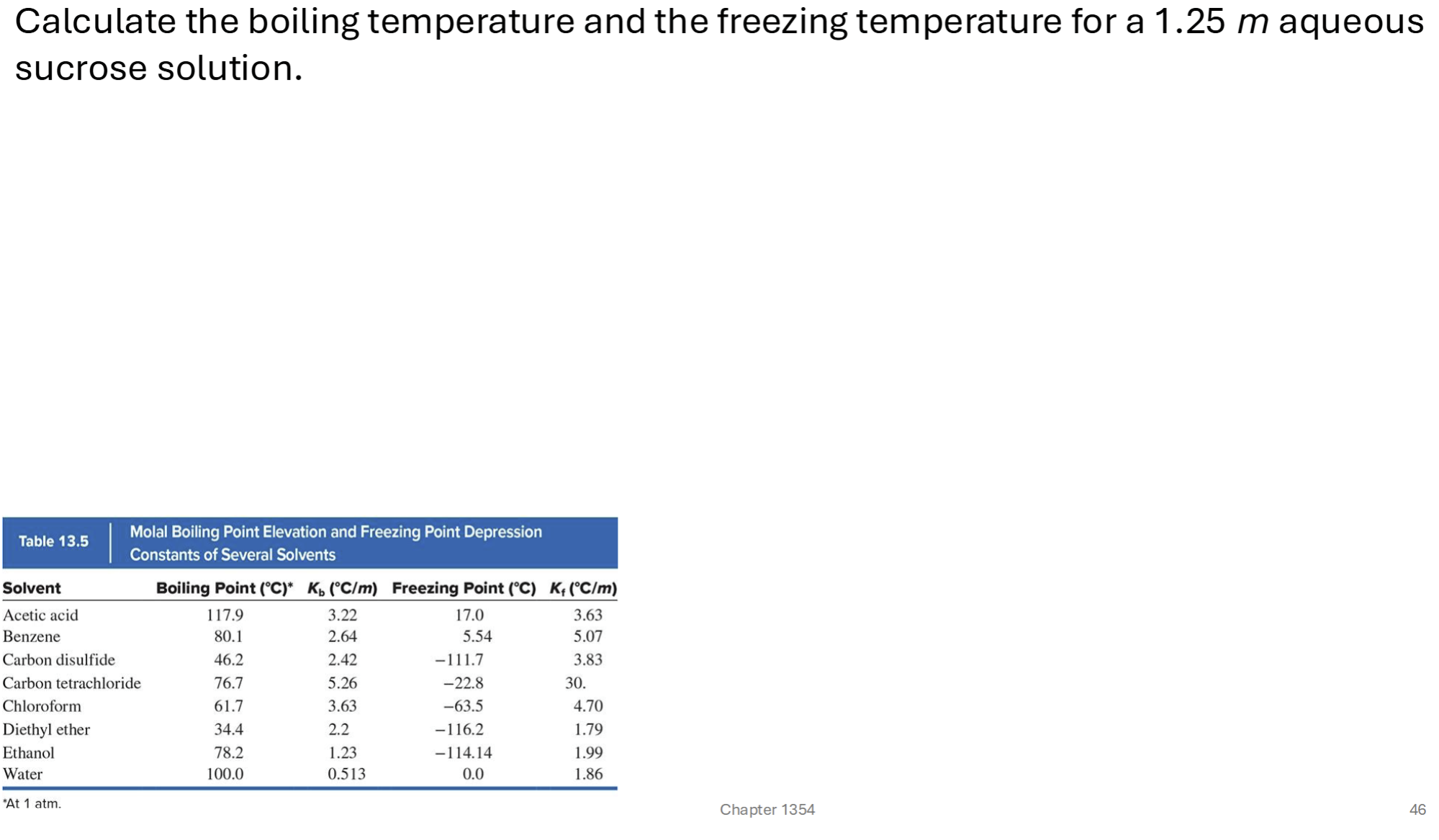
PRACTICE
-2.3 degrees Celsius
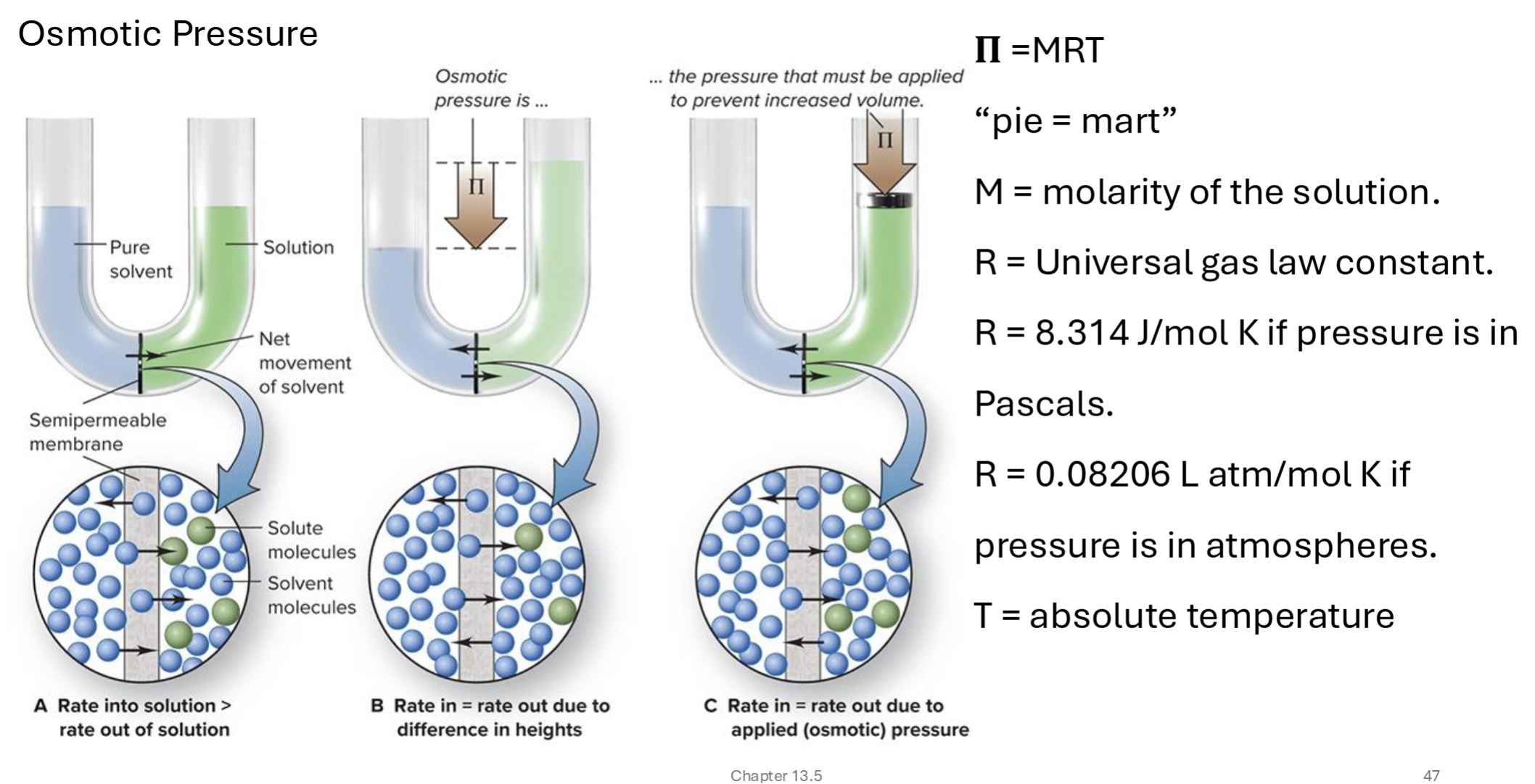
osmotic pressure
pressure to keep solute from going through a semipermeable membrane