Cells and batteries: Energy Changes: Chemistry: GCSE (9:1)
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Electrochemical cell
A device containing chemicals that react to produce electricity
Battery
Two or more electrochemical cells in a series

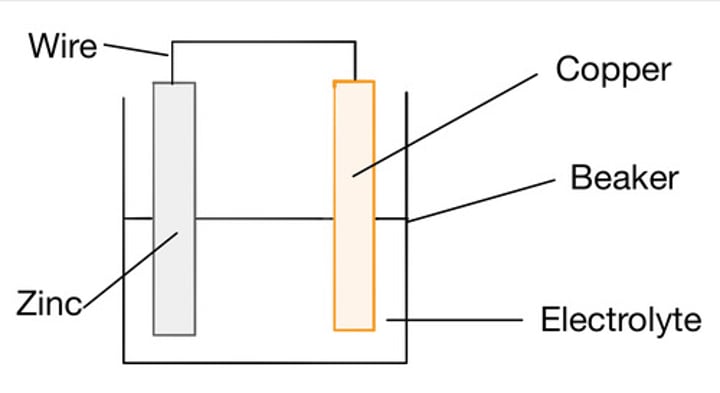
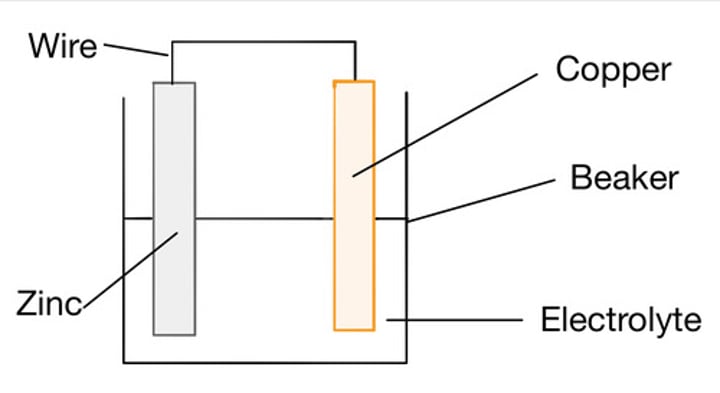
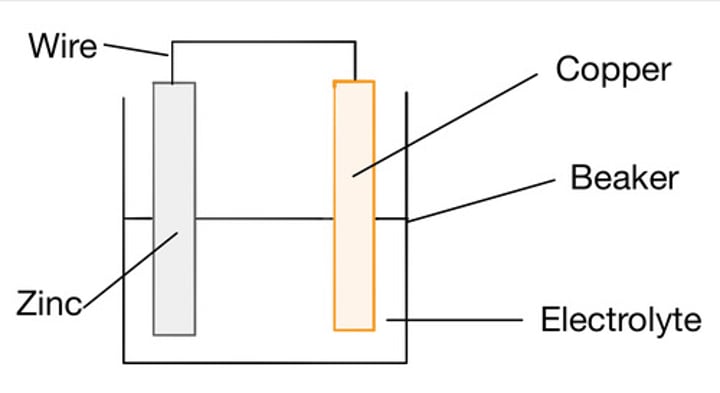
Constructing a simple cell
Join two different metals together, whilst both are in contact with an electrolyte
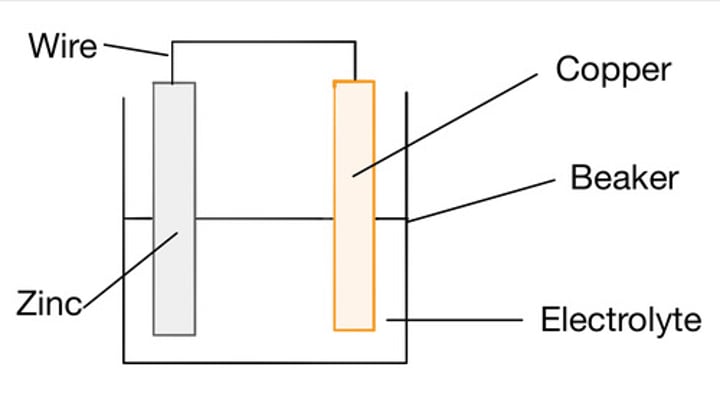
Factors which determine a cell's voltage
Type of electrode, concentration of electrolyte, Type of electrolyte
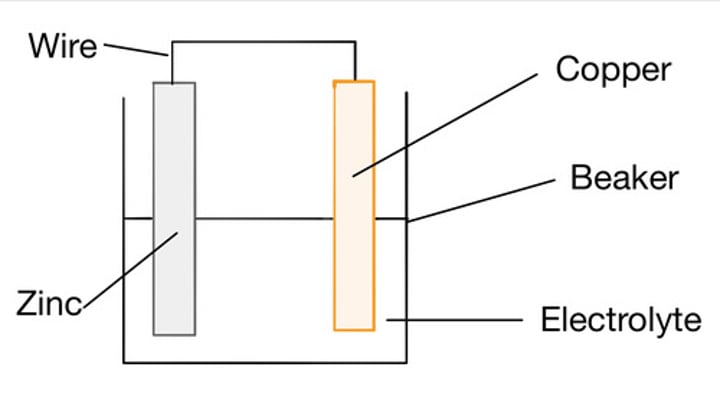
Electrode
a device for conducting electricity
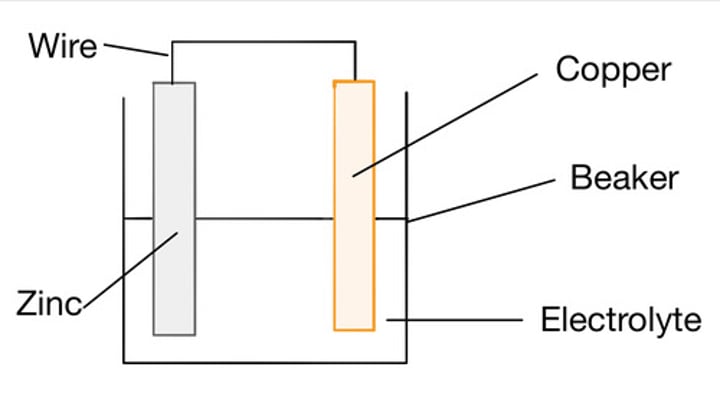
Electrolyte
A substance containing free moving ions
Rechargeable cell
A cell in which the chemical reaction is reversed when a current is applied

Non-rechargeable cell
A cell in which the current stops as a reactant runs out

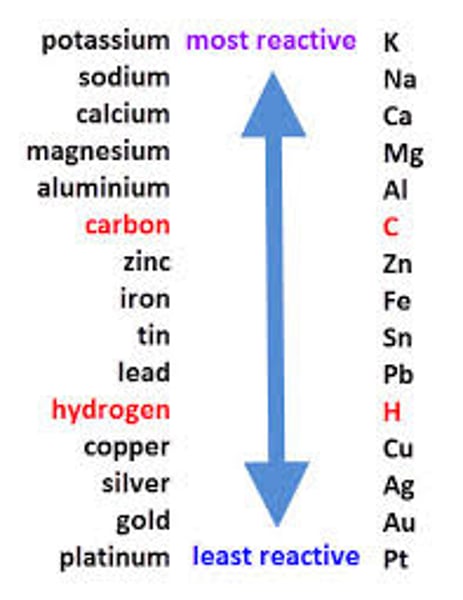
Reactivity and voltage
The larger the difference in electrode reactivity, the higher the voltage
Metal
An element that forms positive ions
Reactivity of a metal
the tendency of a metal to form positive ions
Positive terminal of a cell
Less reactive metal, accepts electrons
Negative terminal of a cell
More reactive metal, gives electrons