Atomic structure
0.0(0)
Card Sorting
1/3
Earn XP
Description and Tags
chemistry for materials and nano technology
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
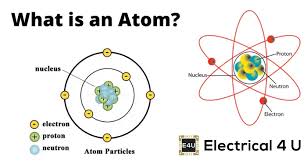
Atoms
The basic building blocks of all matter, consisting of a nucleus (containing protons and neutrons) surrounded by electrons.
2
New cards
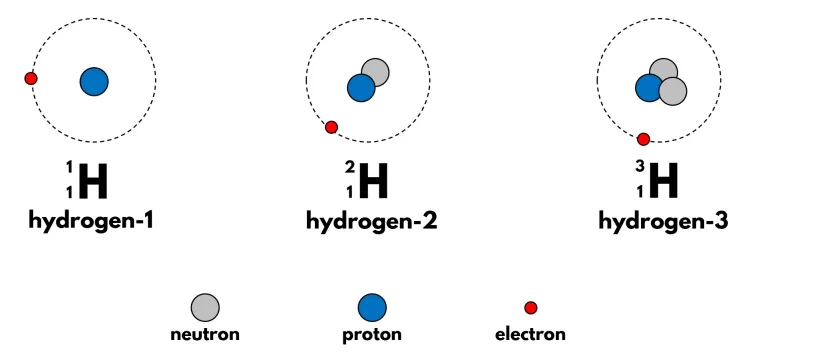
Isotopes
Atoms of the same element that have the same number of protons but different numbers of neutrons, resulting in different mass numbers.
3
New cards
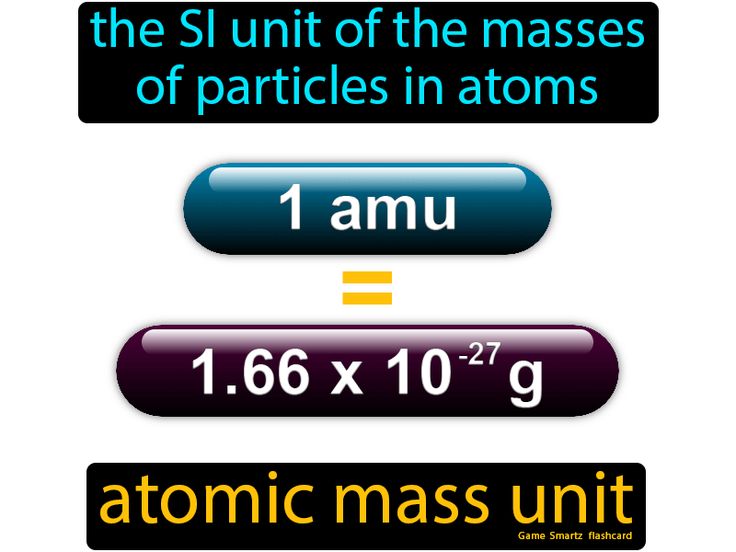
Atomic mass units
A standard unit for expressing the mass of atoms, molecules, and subatomic particles.
4
New cards
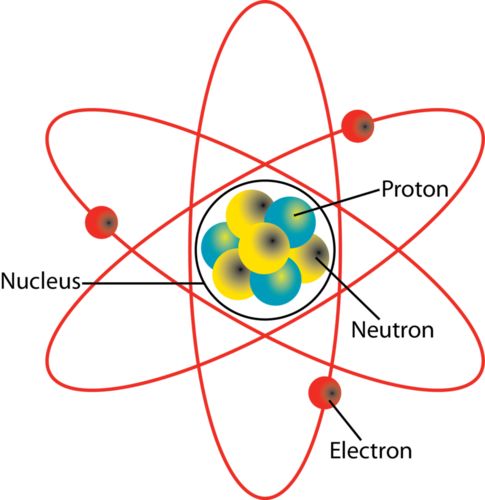
Atomic mass
the number of protons and neutrons in the atom's nucleus