(Unfinished) AQA AS Chemistry
1/138
Earn XP
Description and Tags
This set will (hopefully) contain everything you need to know for AQA AS Chemistry, however currently it is not finished. Note that there are various skills you should be able to do in the exam that can’t really be translated into flashcards, so I would recommend that you do practice questions and/or past papers in addition to using this. I will add A2 content in Year 13 if I continue to take chemistry. | Physical chemistry: Atomic structure: Cards 1-42 Amount of substance: (not started) Bonding: Cards 43-120 Energetics: (not started) Kinetics: Cards 121-137 Chemical equilibria, Le Chatelier’s principle and Kc: (not started) Oxidation, reduction and redox equations: (not started) | Inorganic chemistry: Periodicity: (not started) Group 2, the alkaline earth metals: (not started) Group 7(17), the halogens: (not started) | Organic chemistry: Introduction to organic chemistry: (not started) Alkanes: (not started) Halogenoalkanes: (not started) Alkenes: (not started) Alcohols: (not started) Organic analysis: (not started) | Mathematical requirements: (not started) Practical skills: (not started) | Note: The very first line in the subject content is “Appreciate that knowledge and understanding of atomic structure has evolved over time”. To me, this is not clear at all as to how much you need to know about various models of the atom. I have included one card which simply rephrases that sentence, and a few more which cover the content regarding the development of the model of the atom needed for AQA GCSE chemistry (or physics). You can choose whether or not to study those cards based on how likely you think it is that they could be assessed, because I honestly don’t know.
Chemistry
Atoms
aqa
a level
as
chemistry
science
physical
inorganic
organic
required practical
physical chemistry
atomic structure
fundamental particles
mass number and isotopes
electron configuration
amount of substance
relative atomic mass and relative molecular mass
the mole and the Avogadro constant
the ideal gas equation
empirical and molecular formula
balanced equations and associated calculations
bonding
ionic bonding
nature of covalent and dative covalent bonds
metallic bonding
bonding and physical properties
shapes of simple molecules and ions
bond polarity
forces between molecules
energetics
enthalpy change
calorimetry
applications of Hess’s law
bond enthalpies
kinetics
collision theory
Maxwell-Boltzmann distribution
effect of temperature on reaction rate
effect of concentration and pressure
catalysts
chemical equilibria, Le Chatelier’s principle and Kc
chemical equilibria and Le Chatelier’s principle
equilibrium constant Kc for homogeneous systems
oxidation, reduction and redox equations
inorganic chemistry
periodicity
classification
physical properties of Period 3 elements
Group 2, the alkaline earth metals
Group 7(17), the halogens
trends in properties
uses of chlorine and chlorate(I)
organic chemistry
introduction to organic chemistry
nomenclature
reaction mechanisms
isomerism
alkanes
fractional distillation of crude oil
modification of alkanes by cracking
combustion of alkanes
chlorination of alkanes
halogenoalkanes
nucleophilic substitution
elimination
ozone depletion
alkenes
structure, bonding and reactivity
addition reactions of alkenes
addition polymers
alcohols
alcohol production
oxidation of alcohols
elimination
organic analysis
identification of functional groups by test-tube reactions
mass spectrometry
infrared spectroscopy
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
139 Terms
Has knowledge and understanding of atomic structure stayed the same, or evolved over time?
Evolved over time
What was thought about atoms before the discovery of the electron?
They were thought to be tiny spheres that could not be divided
What led to the plum pudding model of the atom?
The discovery of the electron
What did the plum pudding model of the atom suggest?
That the atom is a ball of positive charge with negative electrons embedded in it
How and why did the new evidence from the alpha particle scattering experiment lead to a change in the atomic model?
Most of the alpha particles passed straight through the gold foil without changing direction. This told the scientists that atoms are mainly empty space. Some alpha particles were deflected. This told the scientists that the centre of the atom must have a positive charge - alpha particles are positive, so any alpha particle that comes close to the positive centre of an atom was repelled and changed direction. Some alpha particles simply bounced straight back off the foil. This told the scientists that the mass of an atom must be concentrated in the nucleus. This nuclear model replaced the plum pudding model.
How was the nuclear model of the atom adapted after the alpha particle scattering experiment?
Niels Bohr suggested that electrons orbit the nucleus at specific distances. The theoretical calculations of Bohr agreed with experimental observations.
Later experiments led to the idea that the positive charge of any nucleus could be subdivided into a whole number of smaller particles, each particle having the same amount of positive charge. The name proton was given to these particles.
The experimental work of James Chadwick provided the evidence to show the existence of neutrons within the nucleus. This was about 20 years after the nucleus became an accepted scientific idea.
What is the relative charge of a proton?
+1
What is the relative charge of a neutron?
0
What is the relative charge of an electron?
-1
What is the relative mass of a proton?
1
What is the relative mass of a neutron?
1
What is the relative mass of an electron?
~1/1836
What is the basic structure of an atom?
An atom consists of a nucleus containing protons and neutrons surrounded by electrons.
What letter is used to represent the mass number of an element?
A
What letter is used to represent the atomic (proton) number of an element?
Z
How is the number of protons in an atom or ion determined?
Number of protons = atomic number
How is the number of neutrons in an atom or ion determined?
Number of neutrons = mass number - atomic number
How is the number of electrons in an atom or ion determined?
Number of electrons = atomic number - charge
What are isotopes?
Different atoms of the same element that contain the same number of protons but a different number of neutrons
How are particles ionised in a simple time of flight (TOF) mass spectrometer? Write an equation to represent this process for a general element X.
In electron impact ionisation, the sample is vaporised and then bombarded with high energy electrons fired from an electron gun. An electron is knocked off each particle, forming a 1+ ion. X(g) → X⁺(g) + e⁻
In electrospray ionisation, the sample is dissolved in a volatile solvent. The solvent is injected into the mass spectrometer using a hypodermic needle, producing a fine mist. The needle is attached to a high voltage power supply, so as the sample is injected, the particles are ionised by gaining a proton from the solvent. X(g) + H⁺ → XH⁺(g)
How are ions accelerated in a simple time of flight (TOF) mass spectrometer?
Using an electric field. The ions are all accelerated to have the same kinetic energy.
How are ions detected in a simple time of flight (TOF) mass spectrometer?
After drifting down the flight tube, the ions hit a negatively charged detector and gain an electron. This discharges the ion, and causes a current to be produced. The size of the current is proportional to the abundance of each ion.
What do mass spectra give accurate information about?
Relative isotopic mass and the relative abundance of isotopes
What can the information given in a mass spectrum be used to do?
Identify elements and determine relative molecular mass
What is on the x-axis of a mass spectrum?
The mass-to-charge ratio of the ion
What is on the y-axis of a mass spectrum?
The relative abundance of the ion
What does the number of peaks on a mass spectrum of a monatomic element represent?
The number of isotopes
Why does the mass spectrum of a diatomic element contain more peaks than there are isotopes of that element? How can the relative heights of the peaks be determined (where possible)?
When a molecule of a diatomic element is ionised, a diatomic molecular ion is created. These ions are not very stable, and so some will fragment to create an atom and a monatomic ion. The atoms will not be detected as they have no charge, and the monatomic ions will create peaks in the mass spectrum like those of monatomic elements.
The diatomic ions will create peaks with higher m/z values. The relative heights of these peaks can be determined by considering the probabilities of all the combinations of isotopes that could be present in the diatomic ion.
The relative heights of the peaks corresponding to the monatomic ions cannot be compared with those of the diatomic ions without knowing what proportion of the diatomic ions fragmented.
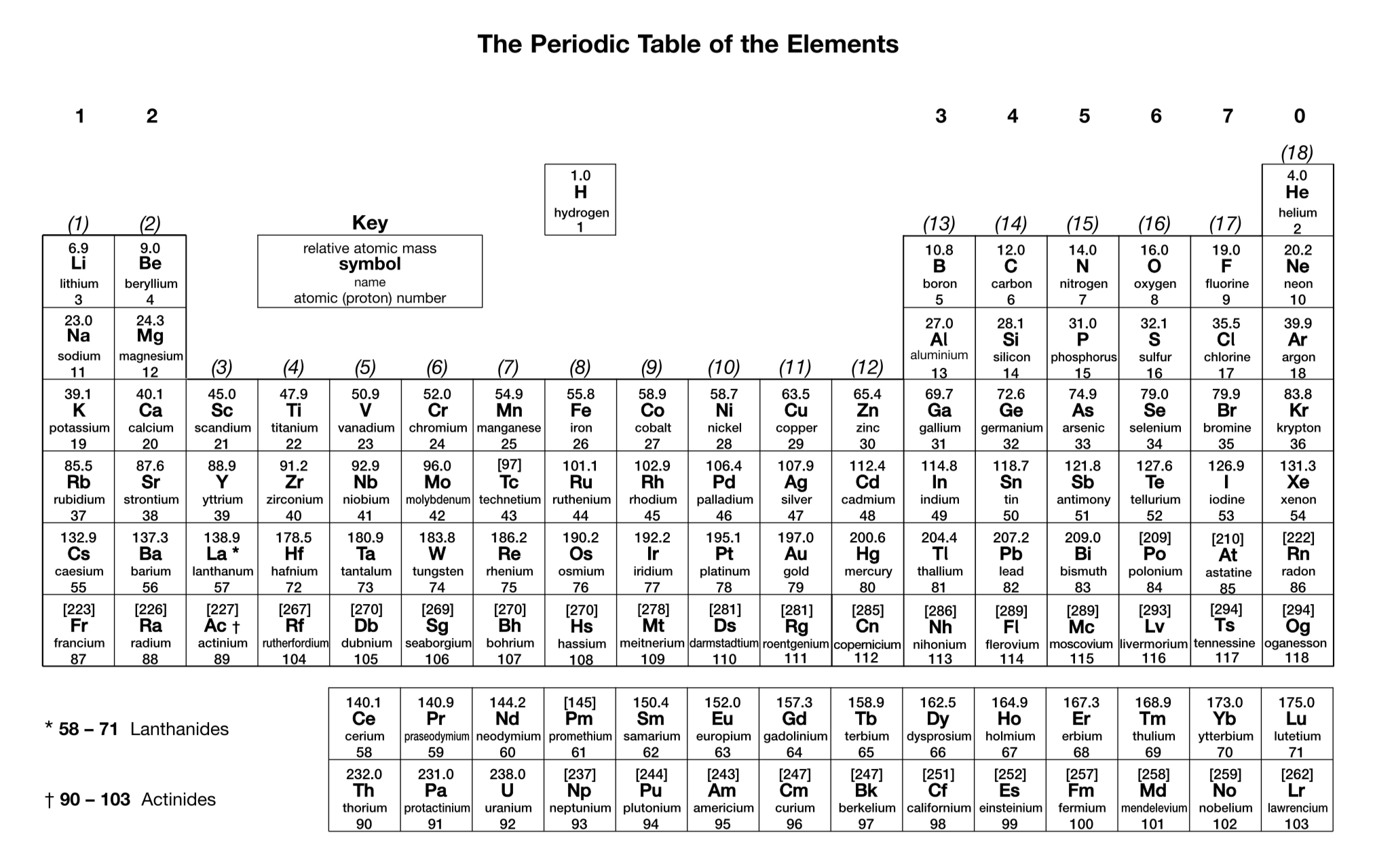
How is relative atomic mass calculated from isotopic abundance?
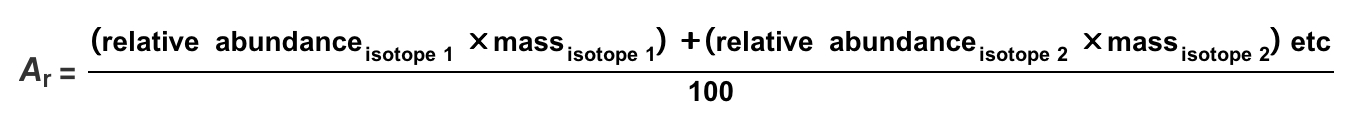
How many orbitals are there in an s sub-shell?
1
How many orbitals are there in a p sub-shell?
3
How many orbitals are there in a d sub-shell?
5
What general rules can be used to work out electron configurations of atoms?
Orbitals with the lowest energy are filled first.
Each orbital can hold up to two electrons with opposite spins.
If there are multiple orbitals with the same energy, electrons are put into individual orbitals before they are paired.
(Note: You don’t need to know these rules word-for-word, but you need to be able to work out electron configurations for atoms up to Z = 36.)
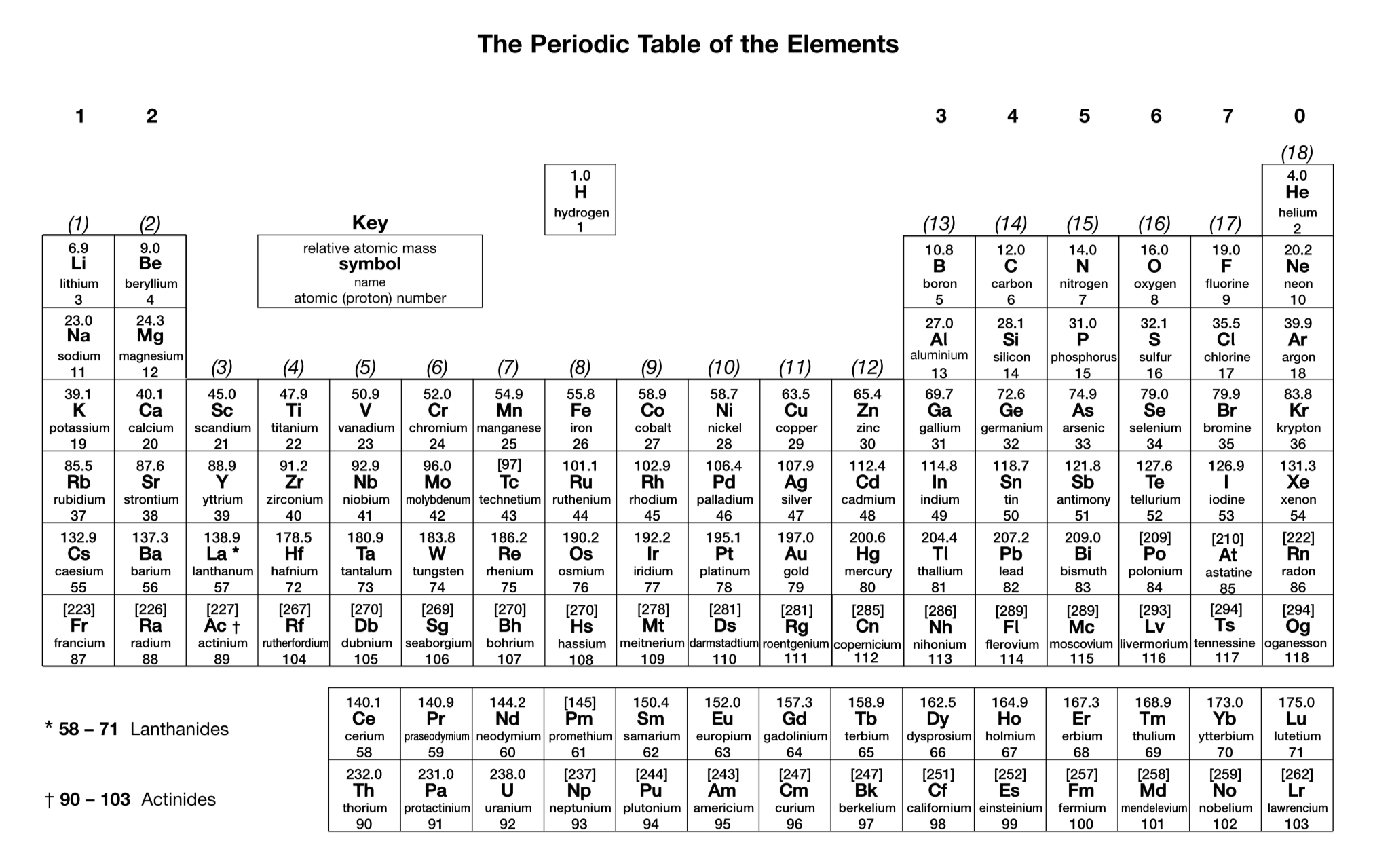
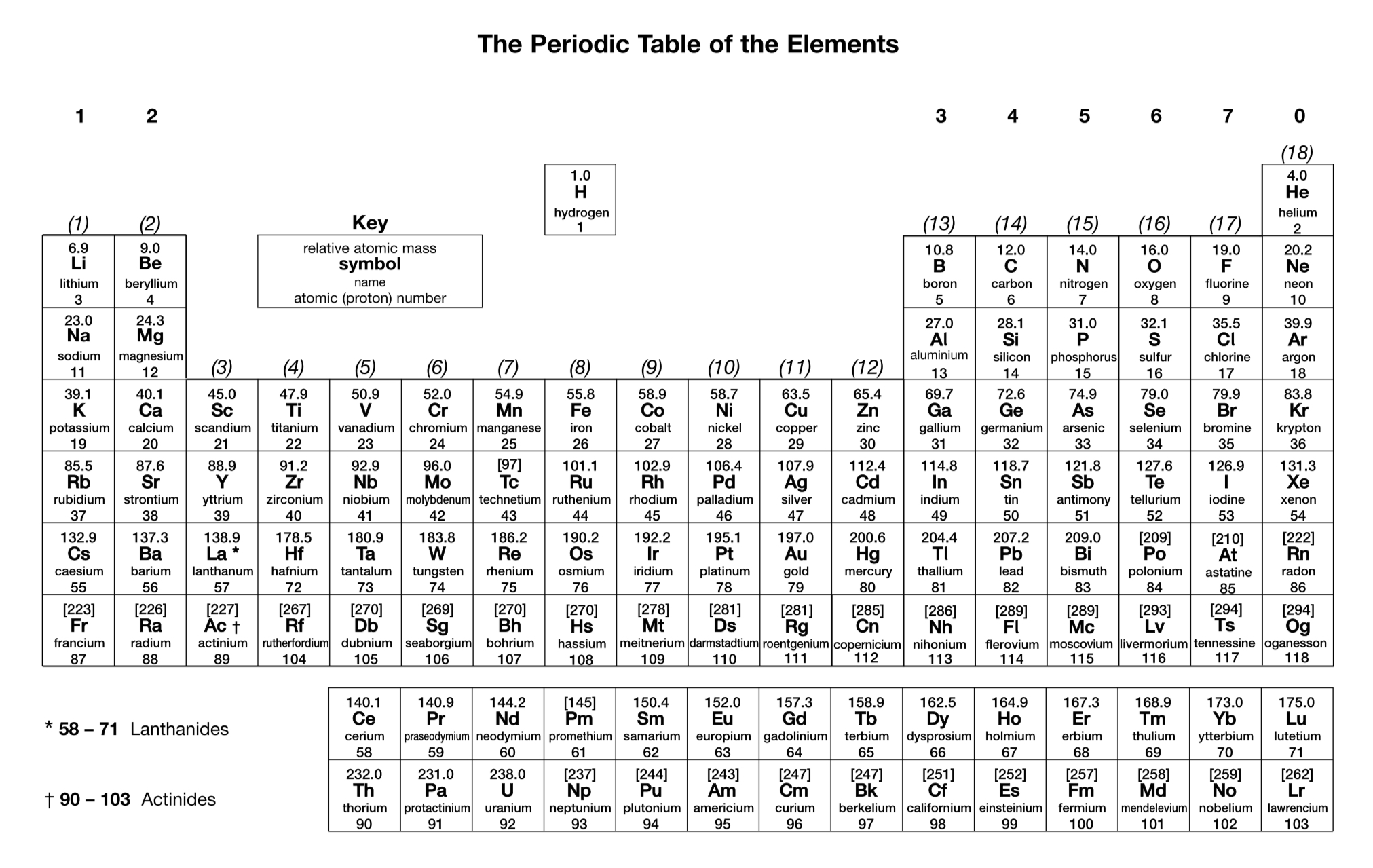
In what order are atomic orbitals filled by electrons (up to Z = 36)?
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p
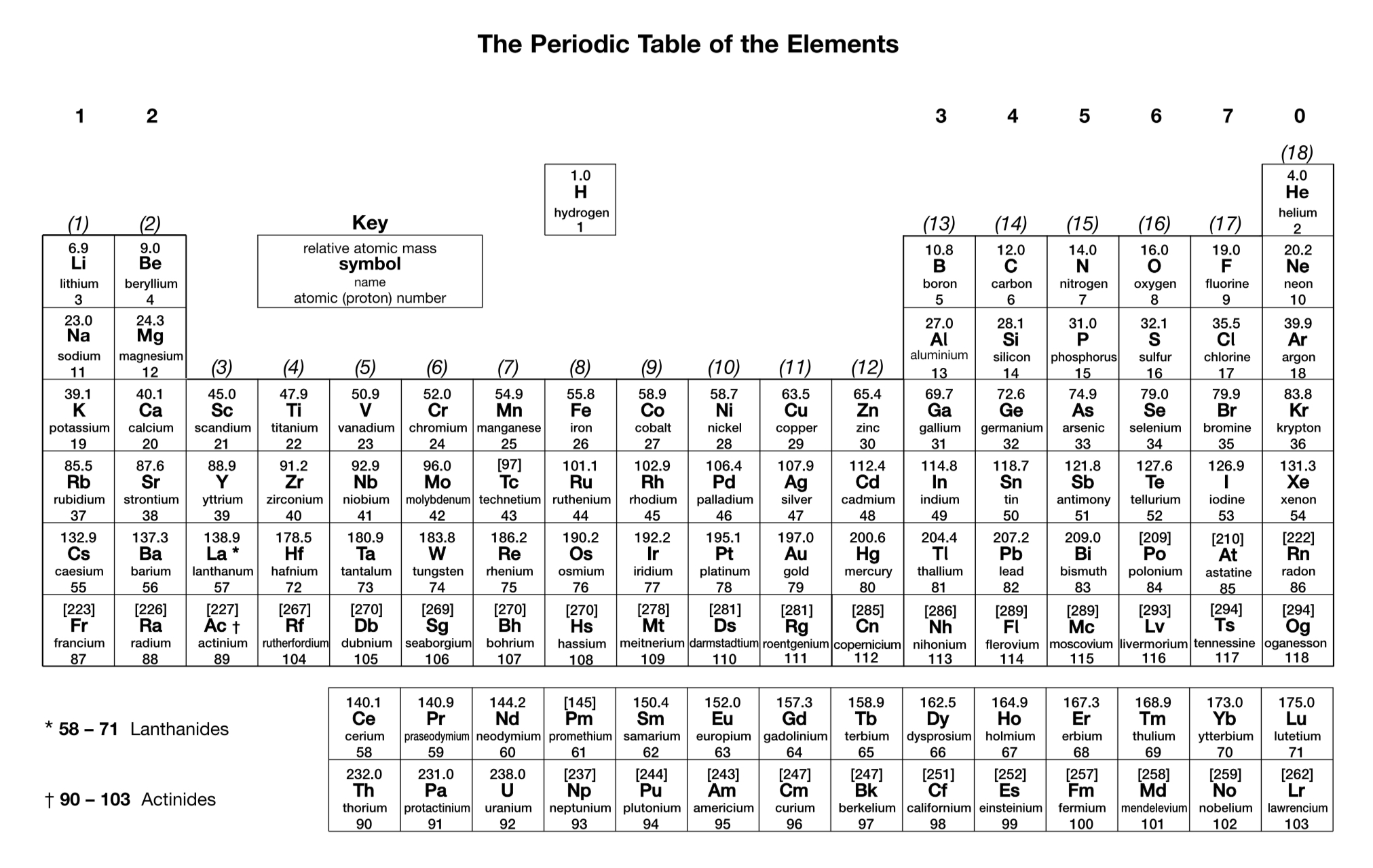
What exceptions are there to the general rules used to work out the electron configurations of atoms (up to Z = 36)? Write the correct electron configurations of the relevant elements.
Chromium: 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁵ 4s¹
Copper: 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s¹
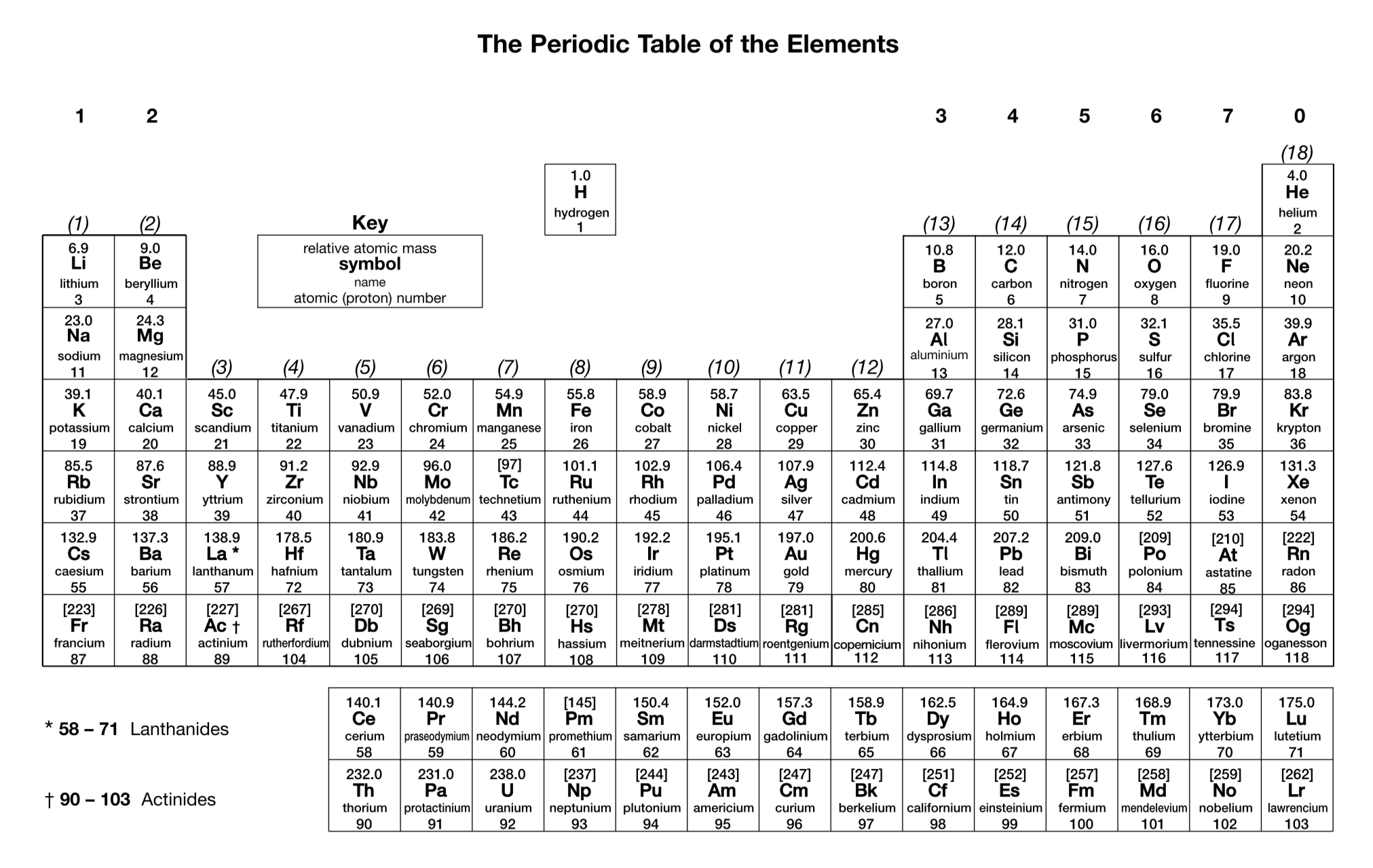
How is the electron configuration of an ion worked out?
Negative ions are formed by adding electrons to the outer sub-shell.
Positive s- and p-block ions are formed by removing electrons from the outer sub-shell.
Positive d-block ions are formed by removing electrons from the 4s sub-shell before the 3d sub-shell.
(Note: You don’t need to know these rules word-for-word, but you need to be able to work out electron configurations for ions up to Z = 36.)
What is first ionisation energy?
The energy needed to remove one mole of electrons from one mole of atoms in their gaseous state to form one mole of 1+ ions in their gaseous state
Write an equation, including state symbols, to represent the process in which the first ionisation energy of a general element X is measured.
X(g) → X⁺(g) + e⁻
Write an equation, including state symbols, to represent the process in which successive ionisation energies of a general element X are measured.
For the nth ionisation energy: X⁽ⁿ ⁻ ¹⁾⁺(g) → Xⁿ⁺(g) + e⁻
How do successive ionisation energies give evidence for electron configuration in shells?
There is a gradual increase in the successive ionisation energies of an element. This is because each time an outer electron is removed, the remaining electrons in the outer shell are pulled slightly closer to the nucleus. This means that there is a greater attraction between the outer electrons and the nucleus, and this causes the ionisation energy to gradually increase.
There is a large increase in ionisation energy when all of the outer shell electrons are removed. This is because the next electron is removed from an electron shell closer to the nucleus, where electrons experience much less shielding. This means that these electrons have a greater attraction to the nucleus compared to the electrons in the old outer shell. This causes the corresponding ionisation energy to be much greater than the previous one.
How do first ionisation energies in Period 3 give evidence for electron configuration in sub-shells?
Moving across Period 3, the nuclear charge increases as the number of protons increases. This increases the attraction between the nucleus and the electrons. Because of this, the atomic radius decreases across a period. Both the increased nuclear charge and the decreased atomic radius mean that the outer electrons are more attracted to the nucleus. This causes the first ionisation energy to increase across the period. In all of these elements, an electron is being removed from the third electron shell, so the shielding effect due to the inner electron shells is similar for each element.
However, there is a decrease in first ionisation energy going from magnesium to aluminium. For magnesium, an electron is being removed from the 3s sub-shell. However, for aluminium, the outer electron is now in the 3p sub-shell, which has a higher energy than the 3s sub-shell. This means that it takes less energy to remove the outer electron of aluminium compared to the outer electron of magnesium. This is why aluminium has a lower first ionisation energy than magnesium.
There is also a decrease in first ionisation energy going from phosphorus to sulfur. In phosphorus, each electron is in a separate 3p orbital. However, in sulfur, one of the orbitals contains a pair of electrons, which repel each other. This means that it takes less energy to remove one of these electrons than if the electrons were in separate orbitals. So because of this, the first ionisation energy of sulfur is less than phosphorus.
How do first ionisation energies in Group 2 give evidence for electron configuration in shells?
The first ionisation energy decreases going down Group 2. This is because, moving down a group, the atomic radius increases. This means that the outer electron shell is further away from the nucleus. The number of internal energy levels also increases. This means that there is more shielding between the nucleus and the outer electrons. Both of these factors mean that going down a group, the attraction between the nucleus and the outer electrons decreases. This causes the first ionisation energy to decrease. Although the nuclear charge increases moving down a group, this is offset by the two aforementioned factors.
What is ionic bonding?
Electrostatic attraction between oppositely charged ions in a lattice
What is the formula of a sulfate ion?
SO₄²⁻
What is the formula of a hydroxide ion?
OH⁻
What is the formula of a nitrate ion?
NO₃⁻
What is the formula of a carbonate ion?
CO₃²⁻
What is the formula of an ammonium ion?
NH₄⁺
What is the charge on a hydrogen ion?
1+
What is the charge on a simple ion of a Group 1 element?
1+
What is the charge on a simple ion of a Group 2 element?
2+
What is the charge on an aluminium ion?
3+
What is the charge on a silver ion?
1+
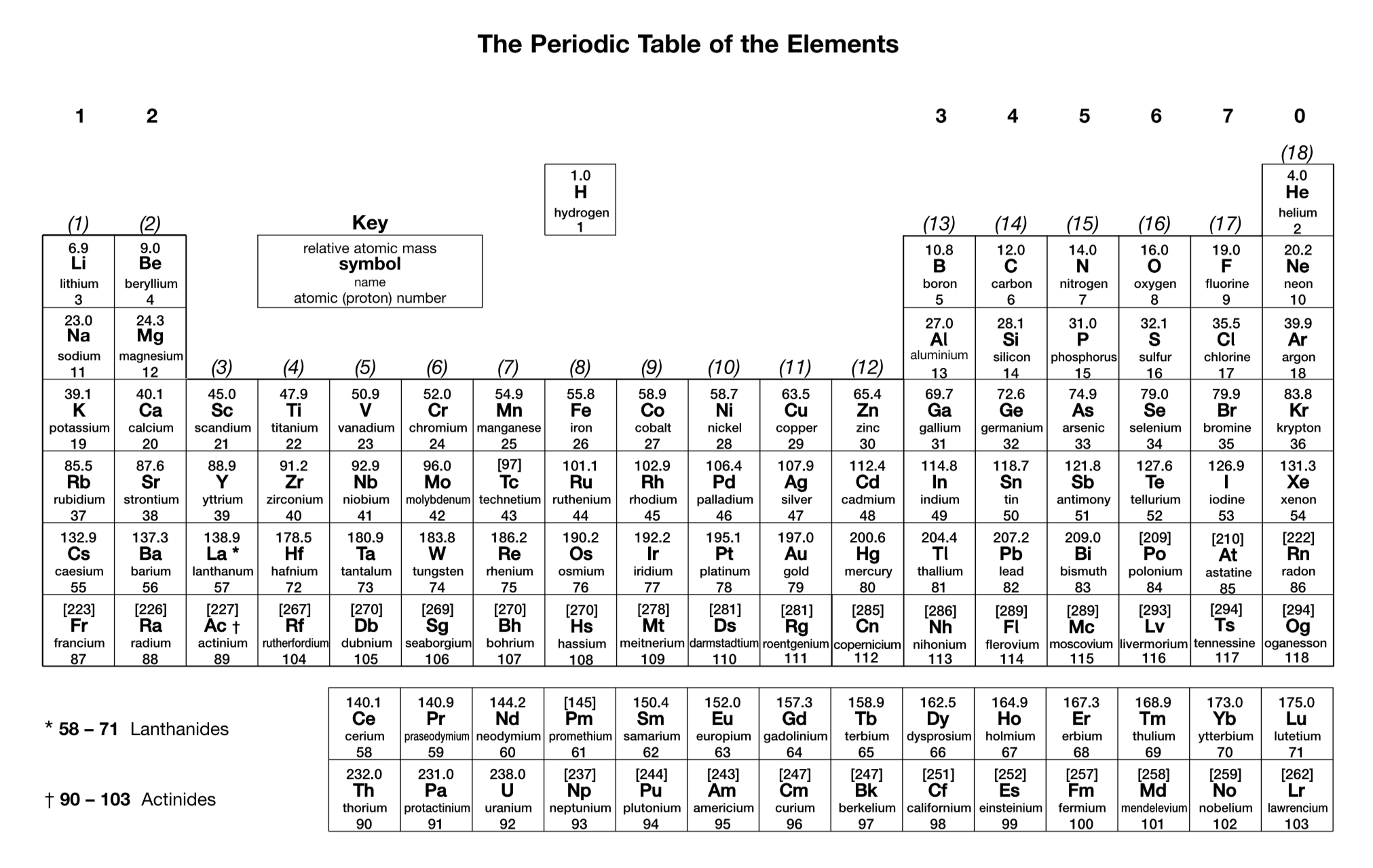
What is the charge on a zinc ion?
2+
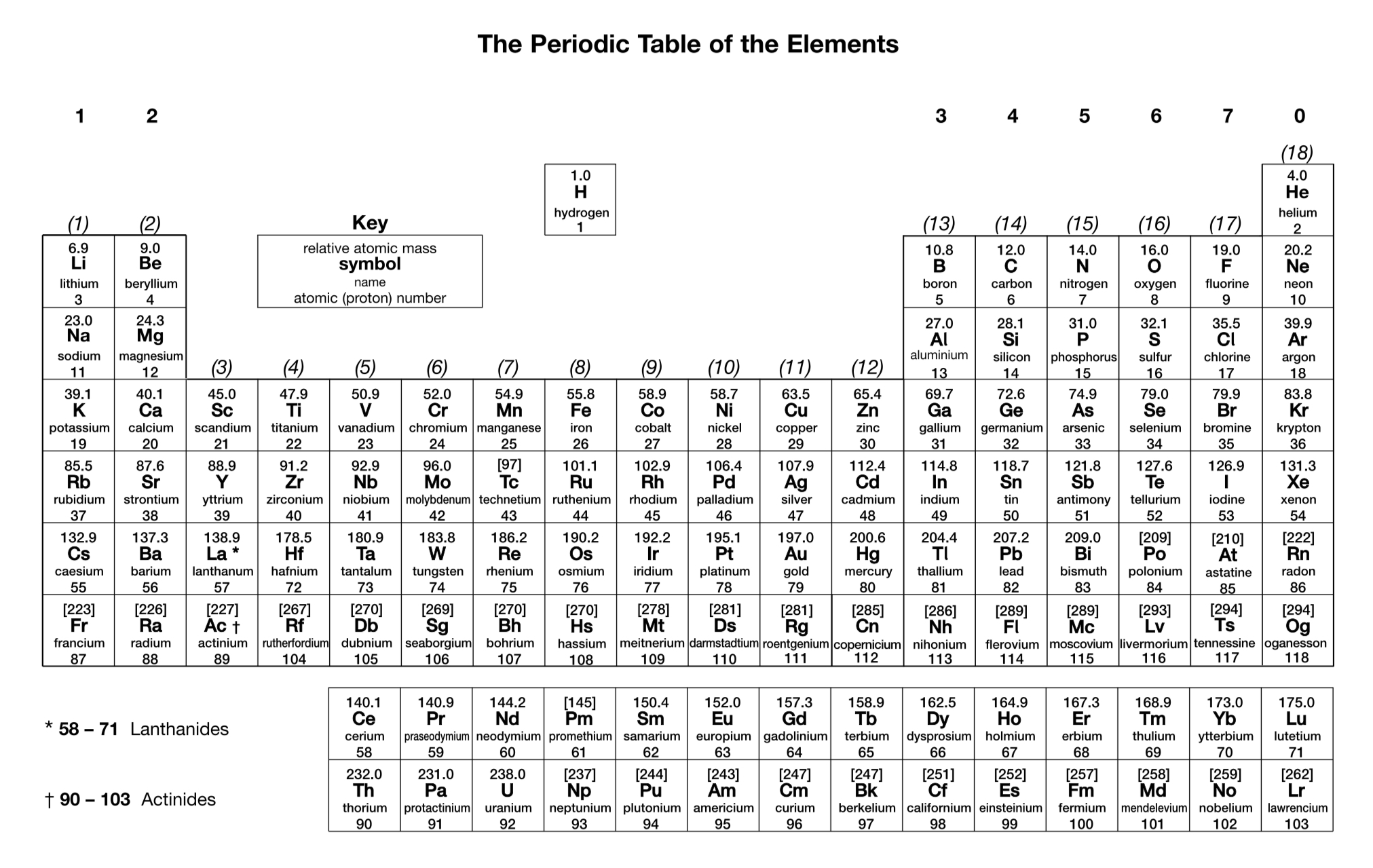
What is the charge on a nitride ion?
3-
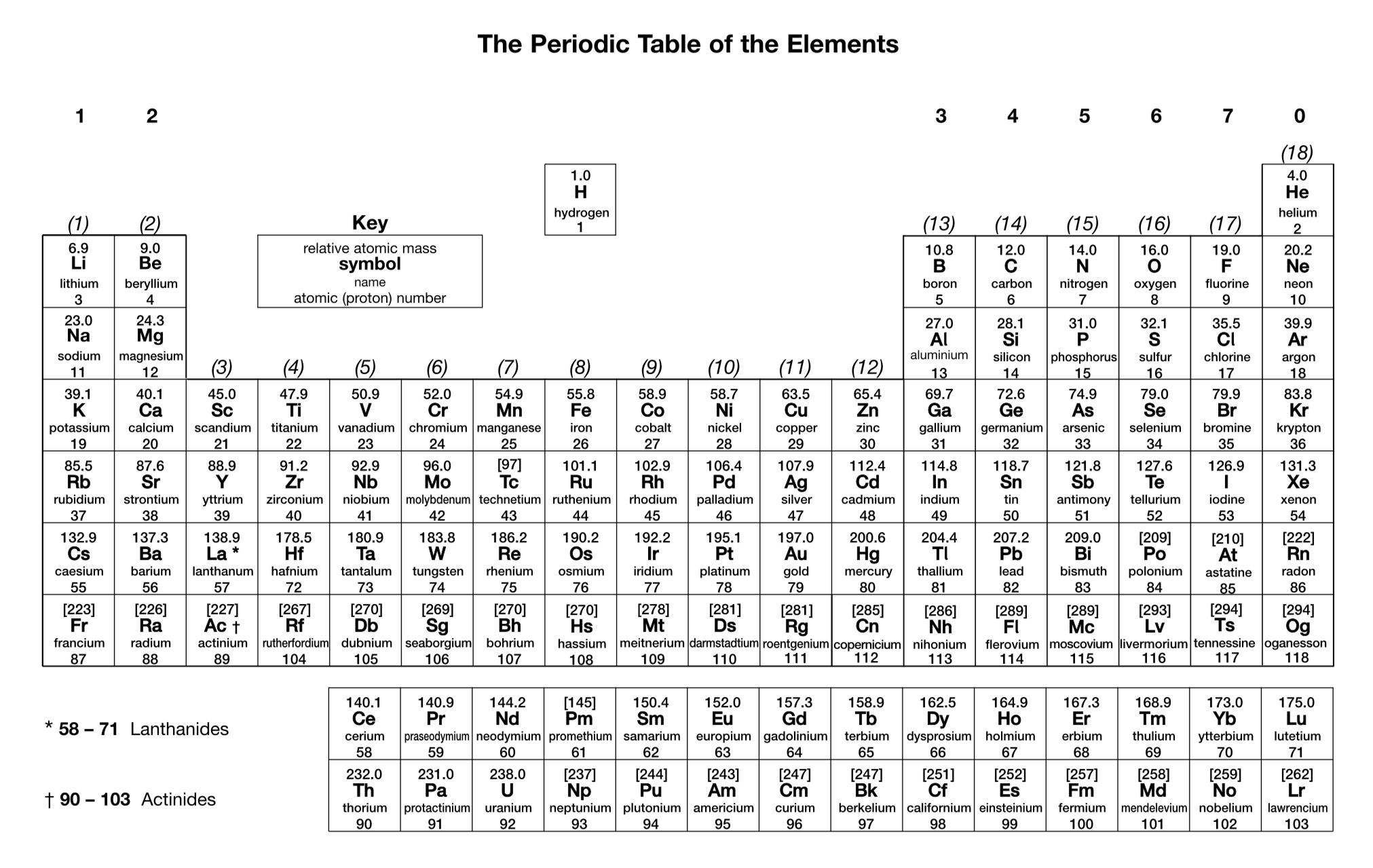
What is the charge on a phosphide ion?
3-
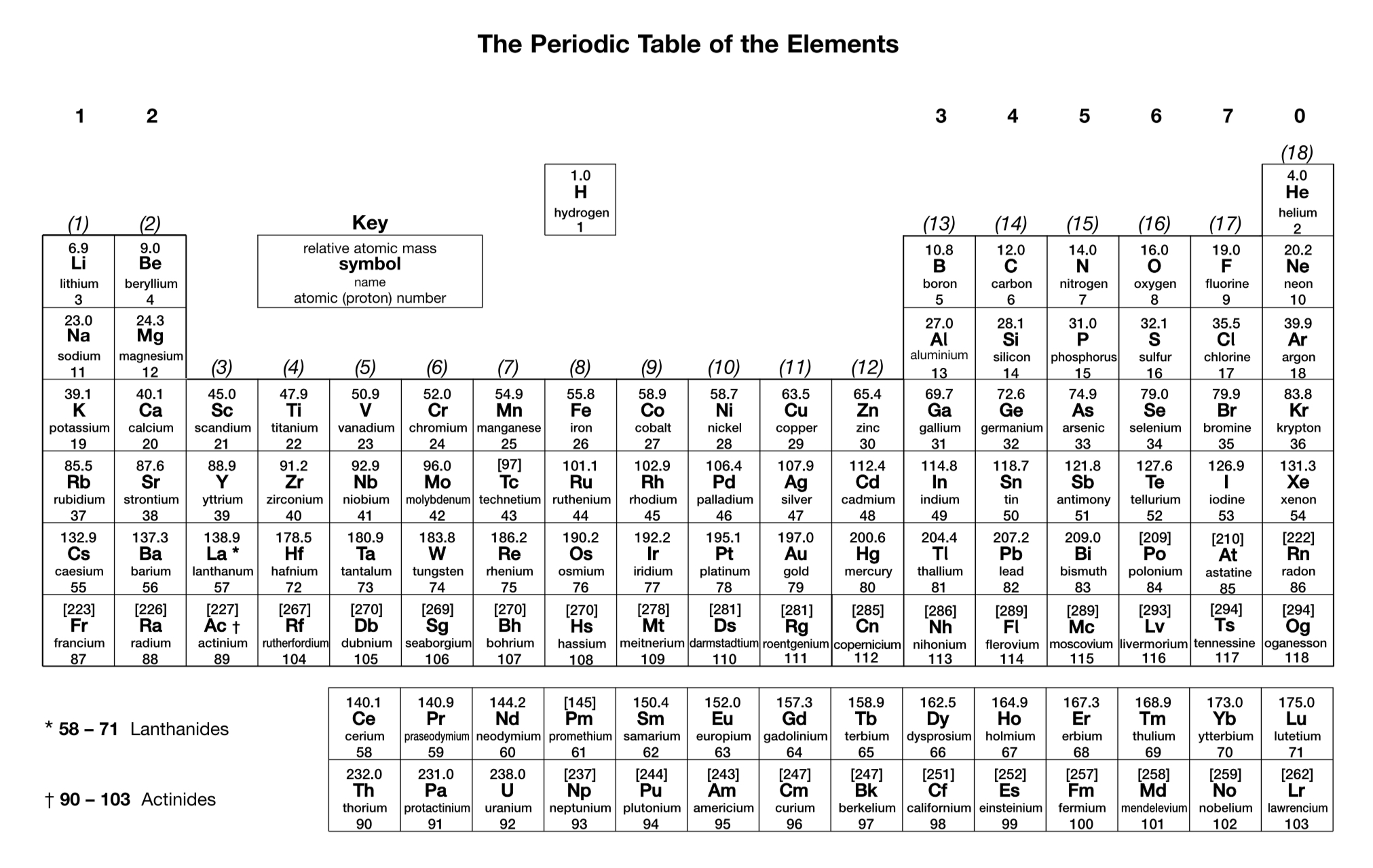
What is the charge on an oxide ion?
2-
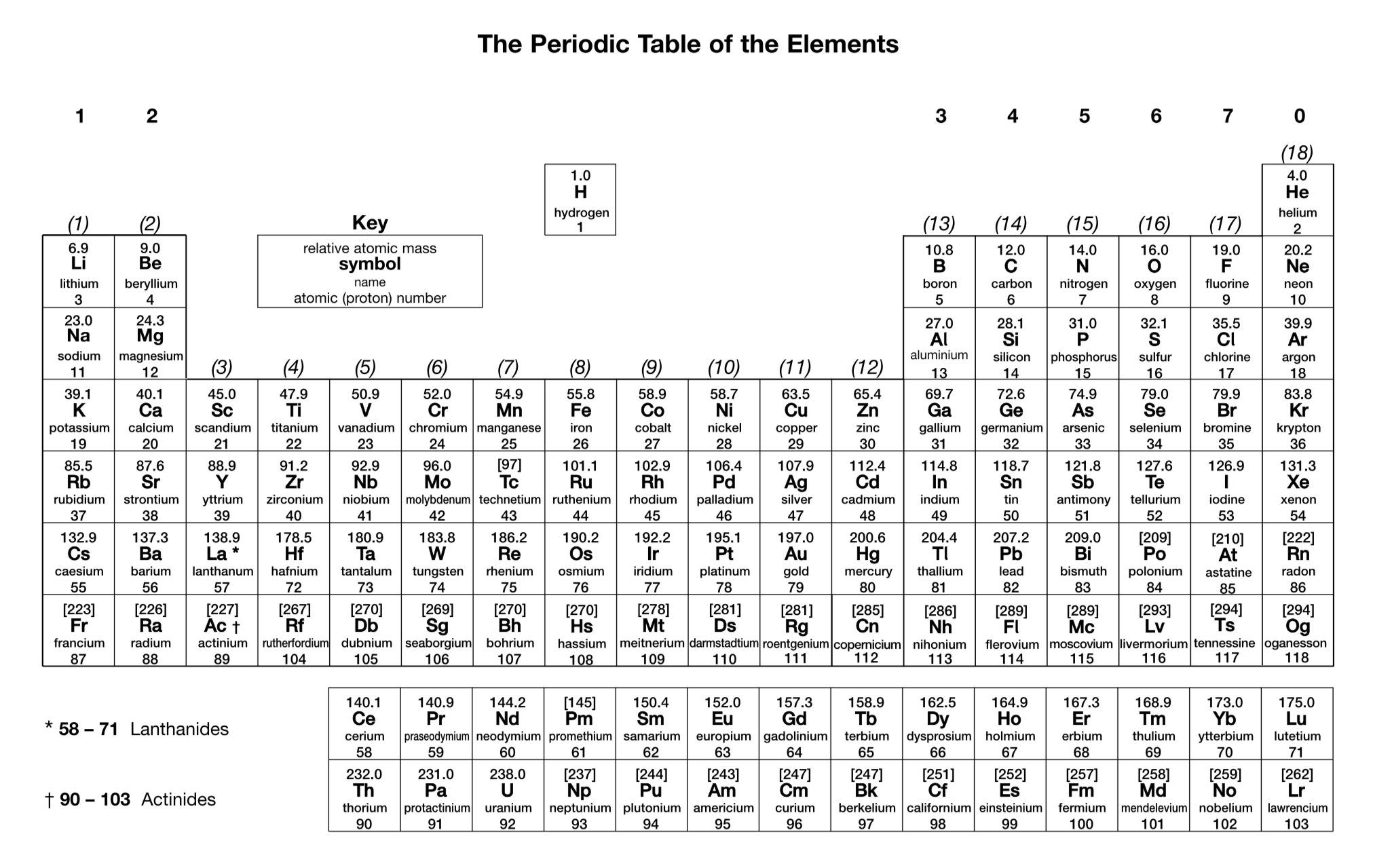
What is the charge on a sulfide ion?
2-
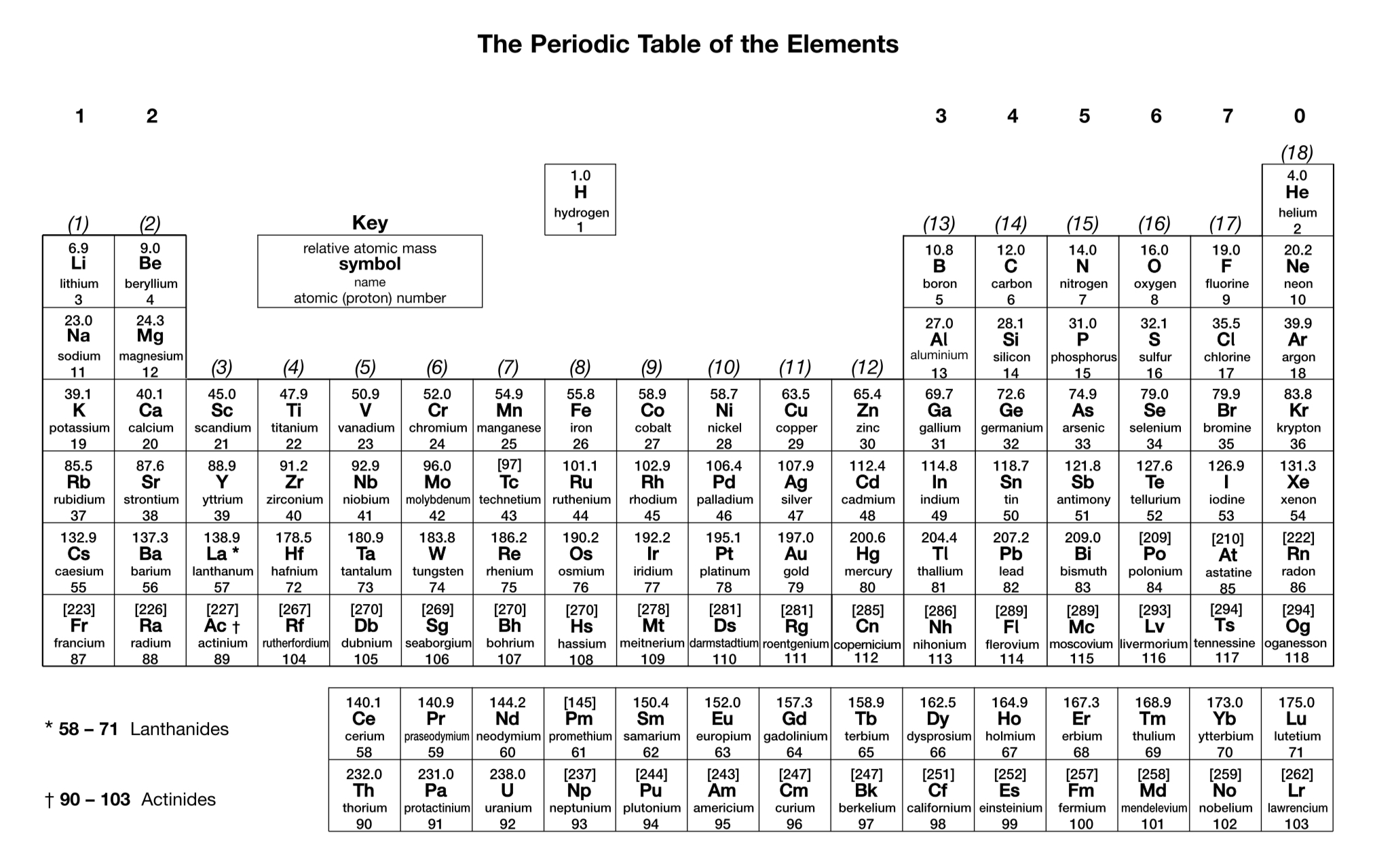
What is the charge on a simple ion of a Group 7 element?
1-
How can formulas for ionic compounds be constructed?
By writing the two ions together (without their charges) in the right ratio, so that their charges cancel out to produce an overall charge of zero
What does a covalent bond contain?
A shared pair of electrons
What does a co-ordinate (dative covalent) bond contain?
A shared pair of electrons with both electrons supplied by one atom
How are covalent bonds represented in diagrams?
Using a line
How are co-ordinate bonds represented in diagrams?
Using an arrow
What is metallic bonding?
Attraction between delocalised electrons and positive ions arranged in a lattice
What are the four types of crystal structure?
Ionic, metallic, macromolecular (giant covalent) and molecular
Explain the melting points of ionic compounds.
Ionic compounds have very high melting points. This is because it takes a large amount of energy to overcome the strong electrostatic forces of attraction between oppositely charged ions in the giant ionic lattice.
Explain the conductivity of ionic compounds.
Ionic compounds do not conduct electricity when they are solids, because the ions are locked in place by the electrostatic forces of attraction acting between oppositely charged ions in the giant ionic lattice, so they cannot carry a charge.
Molten and dissolved ionic compounds can conduct electricity, because the ions are free to move and act as charge carriers.
Are ionic compounds soluble in water?
Often
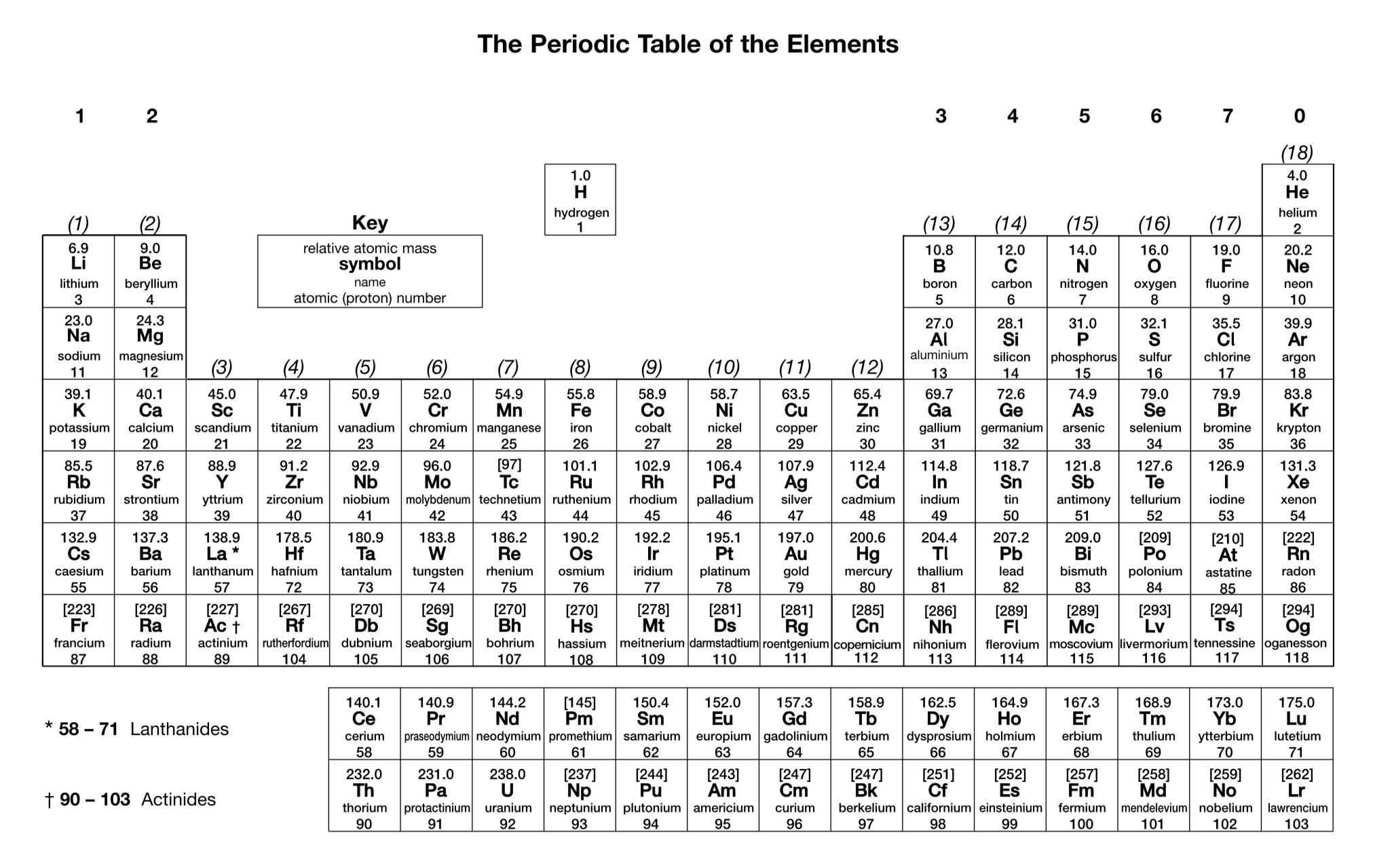
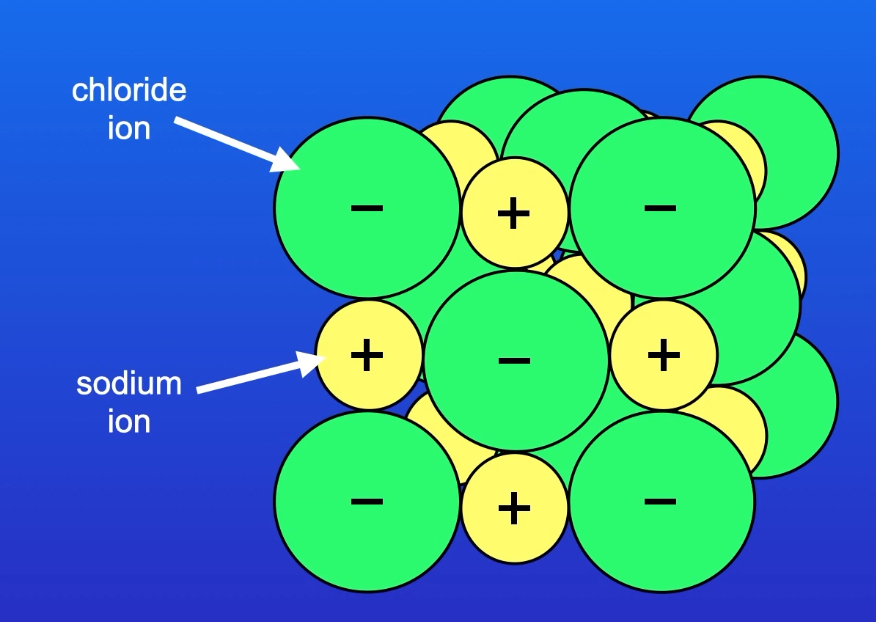
Draw a diagram to represent the structure of sodium chloride crystal.
Explain the melting points of metals.
Most metals have relatively high melting and boiling points, because the electrostatic forces of attraction between the metal cations and delocalised electrons are very strong. It takes a lot of energy to overcome this attraction.
Explain the conductivity of metals.
Metals are good conductors of electricity when they are both solids or liquids, because the delocalised electrons are free to move and act as charge carriers.
Are metals soluble in water?
No
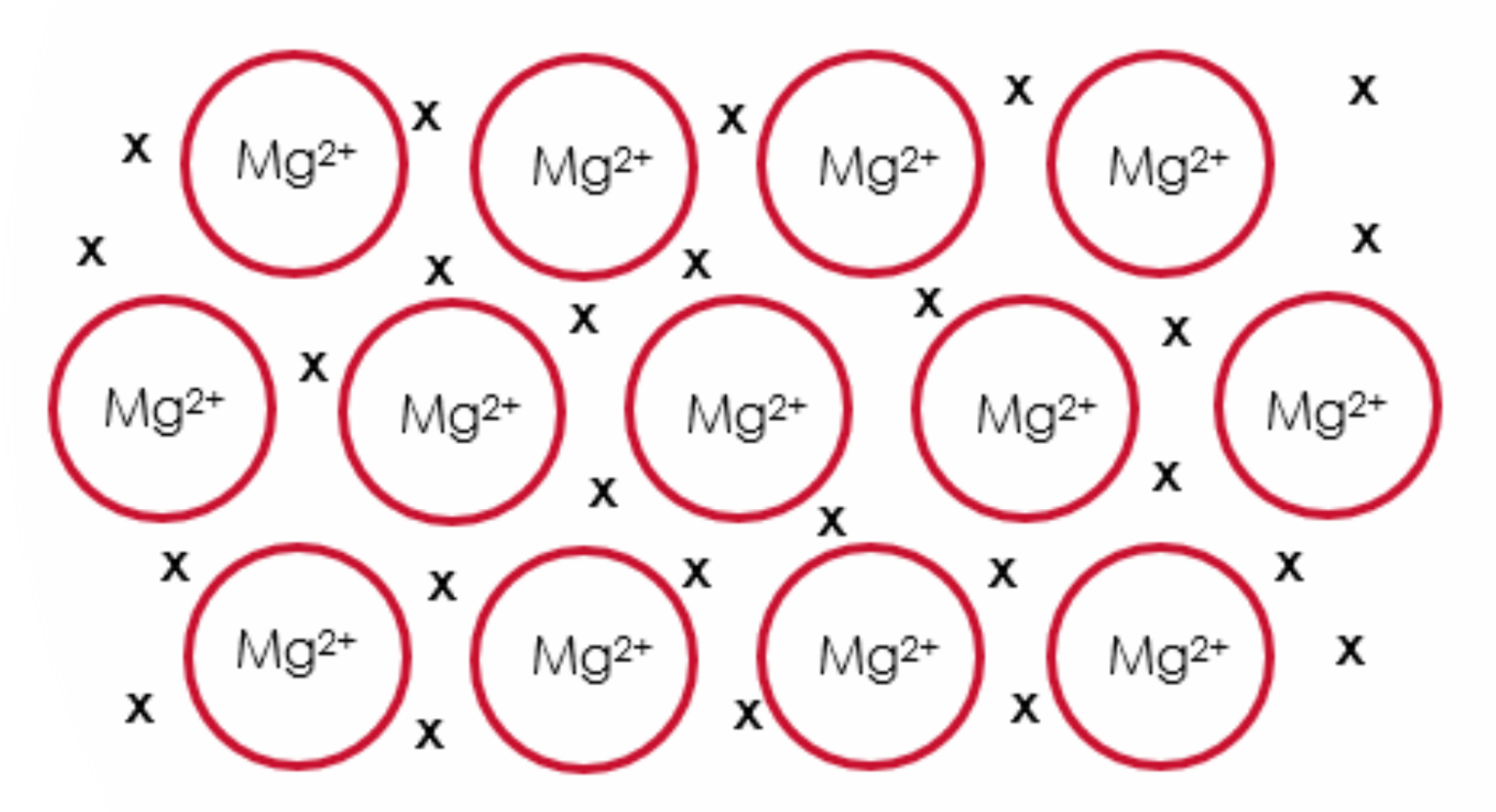
Draw a diagram to represent the structure of magnesium crystal.
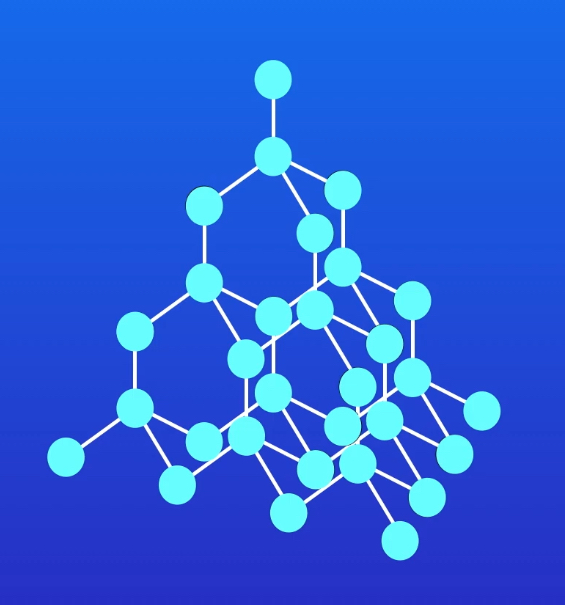
Describe the structure of diamond.
Carbon atoms covalently bond to four other carbon atoms, forming a giant covalent lattice. The carbon atoms are arranged in a tetrahedral structure, with a bond angle of 109.5°.
Explain the melting point of diamond.
Diamond has a high melting point, because the covalent bonds between the carbon atoms are very strong. A large amount of energy is required to break these bonds.
Explain the conductivity of diamond.
Diamond does not conduct electricity, because in every carbon atom, all of the outer electrons are in a covalent bond, so there are no delocalised electrons to act as charge carriers.
Is diamond soluble in water?
No
Draw a diagram to represent the structure of diamond crystal.
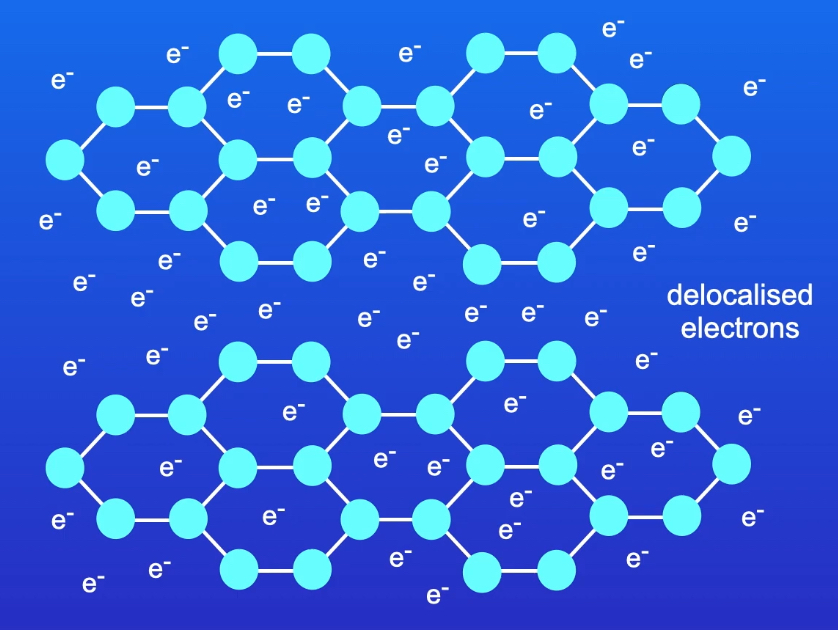
Describe the structure of graphite.
Carbon atoms covalently bond to three other carbon atoms, forming a giant covalent lattice. The carbon atoms form layers of planar hexagonal structures, with a bond angle of 120°. One electron from each carbon atom is delocalised.
Explain the melting point of graphite.
Graphite has a high melting point, because the covalent bonds between the carbon atoms are very strong. A large amount of energy is required to break these bonds.
Explain the conductivity of graphite.
Graphite can conduct electricity, because one electron from each carbon atom is delocalised and can act as a mobile charge carrier.
Is graphite soluble in water?
No
Draw a diagram to represent the structure of graphite crystal.
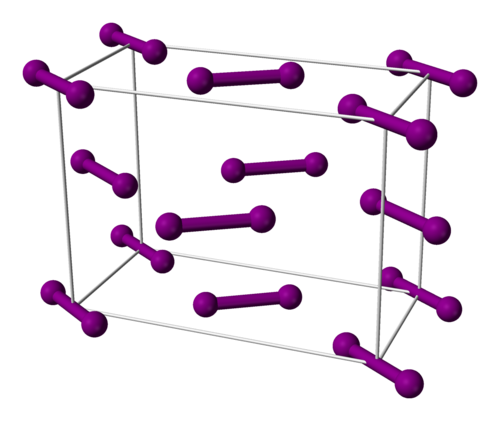
Explain the melting point of iodine.
Because both the iodine atoms in a molecule of iodine have the same electronegativity, the molecule is non-polar. This means that only induced dipole-dipole forces act between the molecules. These are weak and require little energy to break, so iodine has a low melting point.
However, as iodine molecules have more electrons than the other halogens, the induced dipole-dipole forces are stronger in iodine, so iodine has a higher melting point than the other halogens.
Explain the conductivity of iodine.
Iodine does not contain mobile charged particles, so it cannot conduct electricity.
Is iodine soluble in water?
No
Draw a diagram to represent the structure of iodine crystal.
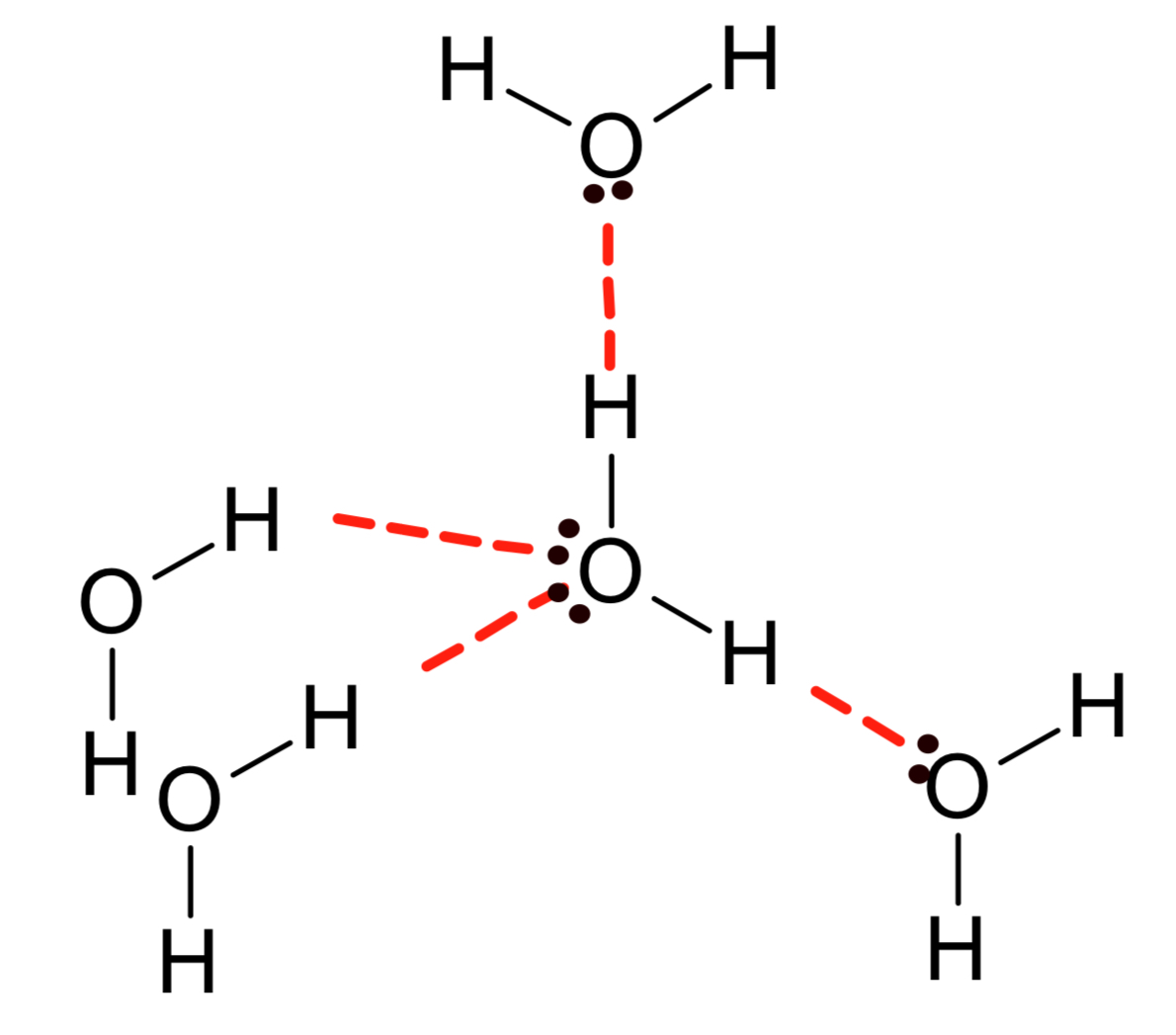
Explain the conductivity of ice.
Ice does not contain mobile charged particles, so it cannot conduct electricity.
Draw a diagram to represent the structure of ice crystal.
Explain the energy changes associated with melting and boiling.
If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a liquid. If more heat energy is supplied, the particles eventually move fast enough to break all the attractions between them, and the liquid boils.
Explain the energy changes associated with evaporation and sublimation.
Some of the more energetic particles on the surface of a liquid can be moving fast enough to escape from the attractive forces holding the liquid together - they evaporate. Solids can also lose particles from their surface to form a vapour - this is sublimation.
Explain the energy changes associated with freezing and condensing.
If a gas is cooled, at some temperature the gas particles will slow down enough for the attractions to become effective enough to condense it back into a liquid. As these forces are re-established, heat energy is released. When a liquid freezes, the motion of the particles is slow enough for the forces of attraction to be able to hold the particles as a solid. As the new bonds are formed, heat energy is released.
How are bonding pairs and lone pairs of electrons modelled when determining the shape of a simple molecule or ion using VSEPR principles?
As charge clouds that repel each other
Draw a diagram to represent a molecule of a hypothetical compound AX₂, which has two bonding pairs and no lone pairs of electrons around the central atom. Explain its shape and bond angle(s).
Pairs of electrons in the outer shell of atoms arrange themselves as far apart as possible to minimise repulsion. The two bonding pairs repel each other equally, giving the molecule a linear shape with a bond angle of 180°.
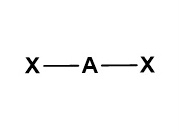
Draw a diagram to represent a molecule of a hypothetical compound AX₃, which has three bonding pairs and no lone pairs of electrons around the central atom. Explain its shape and bond angle(s).
Pairs of electrons in the outer shell of atoms arrange themselves as far apart as possible to minimise repulsion. The three bonding pairs repel each other equally, giving the molecule a trigonal planar shape with a bond angle of 120°.
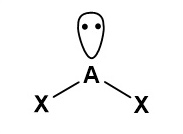
Draw a diagram to represent a molecule of a hypothetical compound AX₂, which has two bonding pairs and one lone pair of electrons around the central atom. Explain its shape and bond angle(s).
Pairs of electrons in the outer shell of atoms arrange themselves as far apart as possible to minimise repulsion. As lone pair–bond pair repulsion is greater than bond pair–bond pair repulsion, the molecule has a bent shape with a bond angle decreased from 120° to slightly less than 120°.
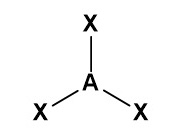
Draw a diagram to represent a molecule of a hypothetical compound AX₄, which has four bonding pairs and no lone pairs of electrons around the central atom. Explain its shape and bond angle(s).
Pairs of electrons in the outer shell of atoms arrange themselves as far apart as possible to minimise repulsion. The four bonding pairs repel each other equally, giving the molecule a tetrahedral shape with a bond angle of 109.5°.
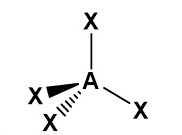
Draw a diagram to represent a molecule of a hypothetical compound AX₃, which has three bonding pairs and one lone pair of electrons around the central atom. Explain its shape and bond angle(s).
Pairs of electrons in the outer shell of atoms arrange themselves as far apart as possible to minimise repulsion. As lone pair–bond pair repulsion is greater than bond pair–bond pair repulsion, the molecule has a trigonal pyramidal shape with a bond angle decreased from 109.5° to approximately 107°.
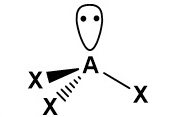
Draw a diagram to represent a molecule of a hypothetical compound AX₂, which has two bonding pairs and two lone pairs of electrons around the central atom. Explain its shape and bond angle(s).
Pairs of electrons in the outer shell of atoms arrange themselves as far apart as possible to minimise repulsion. As lone pair–lone pair repulsion is greater than lone pair– bond pair repulsion, which is greater than bond pair–bond pair repulsion, the molecule has a bent shape with a bond angle decreased from 109.5° to approximately 104.5°.