Chapter 4: Atoms and Elements
4.1: Elements and Symbols
- Elements
- These are pure substances from which all other things are built.
- These cannot be broken down into simpler substances.
- Chemical Symbols
- These are one- or two-letter abbreviations for the names of the elements.
- Only the first letter of an element’s symbol is capitalized.
- If the symbol has a second letter, it is lowercase so that we know when a different element is indicated.
- If two letters are capitalized, they represent the symbols of two different elements.
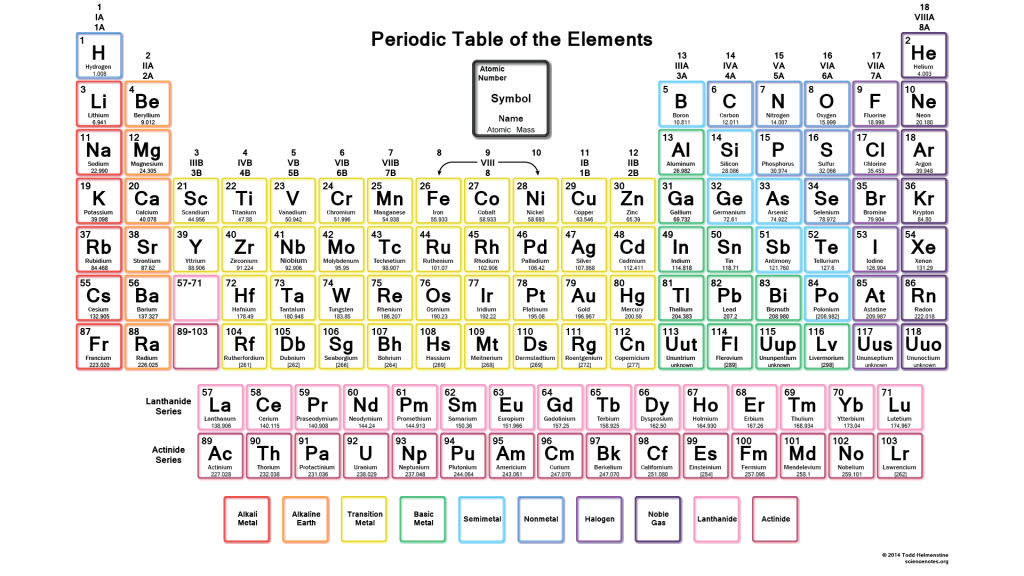
4.2: The Periodic Table
Periodic Table: The arrangement of 118 elements.
Period: Each horizontal row in the periodic table.
Group: Each vertical column on the periodic table.
Representative Elements: These have group numbers 1A to 8A.
Transition Elements: These are block elements in the center of a periodic table that have group numbers followed by the letter ‘B’.
The two rows of 14 elements called the lanthanides and actinides, which are part of Periods 6 and 7, are placed at the bottom of the periodic table to allow them to fit on a page.
Group 1A: These are alkali metals except for Hydrogen.
Group 2A: These are alkaline earth metals; these are shiny metals but not as reactive.
Group 7A: These are halogens; highly reactive and form compounds with most of the elements.
Group 8A: These are noble gases; they are quite unreactive and are seldom found in combination with other elements.
Metals: These are shiny solids, such as copper, gold, and silver. It can be shaped into wires or hammered into a flat sheet.
- They are good conductors of heat and electricity. They usually heat at a higher temperature than nonmetals.
Nonmetals: They are often poor conductors of heat and electricity.
- They have low melting points and low densities.
Metalloids: These are elements that exhibit some properties that are typical of metals and other properties that are characteristic of nonmetals.
- These are semiconductors because they can be modified to function as conductors or insulators.
4.3: The Atom
- Atom: The smallest unit particle of an element that retains the characteristics of that element.
- Subatomic Particles: These are even smaller bits of matter.
- Cathode Rays: These are streams of small particles produced when electricity is applied to a glass tube.
- Protons: These are the atom’s positively charged particles.
- Electrons: These are the atom’s negatively charged particles.
- Neutrons: These are the atom’s neutral particles.
- Nucleus: The center of the atom.
- Atomic Mass Unit: A very small unit of mass.
- It is defined as one-twelfth of the mass of a carbon atom which has a nucleus containing 6 protons and 6 neutrons.
- Dalton: The atomic mass unit.
Dalton’s Atomic Theory
- All matter is made up of tiny particles called atoms.
- All atoms of a given element are similar to one another and different from atoms of other elements.
- Atoms of two or more different elements combine to form compounds. A particular compound is always made up of the same kinds of atoms and always has the same number of each kind of atom.
- A chemical reaction involves the rearrangement, separation, or combination of atoms. Atoms are never created or destroyed during a chemical reaction.
4.4: Atomic Number and Mass Number
- Atomic Number: This is equal to the number of protons in every atom of that element.
- It is the whole number that appears above the symbol of each element on the periodic table.
- Atomic number = number of protons in an atom
- Mass Number: The total number of protons and neutrons in its nucleus.
- It does not appear on the periodic table because it applies to single atoms only.
- Mass number = number of protons + number of neutrons
- If we are given the mass number of an atom and its atomic number, we can calculate the number of neutrons in its nucleus.
- Number of neutrons in a nucleus = mass number - number of protons
4.5: Isotopes and Atomic Mass
- Isotopes: These are atoms of the same element that have the same atomic number but different numbers of neutrons.
- Atomic Symbol: It indicates the mass number in the upper left corner and the atomic number in the lower left corner.
- Because each isotope has a different mass, chemists have calculated an atomic mass for an “average atom,” which is a weighted average of the masses of all the naturally occurring isotopes of that element.
4.6: Electron and Energy Levels
- Electromagnetic spectrum: It shows the arrangement of different types of electromagnetic radiation in order of increasing energy.
- When the light from the Sun passes through a prism, the light separates into a continuous color spectrum, which consists of the colors we see in a rainbow.
- When light from a heated element passes through a prism, it separates into distinct lines of color separated by dark areas called an atomic spectrum.
- In an atom, each electron has a specific energy known as its energy level, which is assigned values called principal quantum numbers.
- An electron can change from one energy level to a higher level only if it absorbs the energy equal to the difference in energy levels.
- When an electron changes to a lower energy level, it emits energy equal to the difference between the two levels.
- If the energy emitted is in the visible range, we see one of the colors of visible light.
- The electron arrangement of an atom gives the number of electrons in each energy level.
4.7: Trends in Periodic Properties
- Period Properties: These consists of the valence electrons in atoms, the trends in atomic size, ionization energy, and metallic character.
- Valence Electrons: These are the electrons in the outermost energy level.
- Electron-Dot Symbol: Also known as a Lewis Structure, represents the valence electrons as dots that are placed on the sides, top, or bottom of the symbol for the element.
- Ionization Energy: A quantity of energy that is required to remove one of the outermost electrons.
- Cation: A positive particle.
- Anion: A negative particle.
- The ionization energy decreases going down a group.
- Going across a period from left to right, the ionization energy increases.
- Metallic Character: An element that loses valence electrons easily.
- It is more prevalent in the elements on the left side of the periodic table and decreases going from left to right across a period.
- The elements on the right side of the periodic table do not easily lose electrons, which means they are the least metallic.
- Atoms at the bottom of any group have more electron levels, which makes it easier to lose electrons