Chem B11: Intro to General, Organic, and Biochemistry
1/156
Earn XP
Description and Tags
Exam 1 (Ch 1-4) Study Guide
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
157 Terms
Chemistry
Is the science that seeks to understand what matter does by studying what atoms and molecules do.
What is Chemistry the study of?
Is the study of composition, structure, properties, and reactions.
Matter
Is anything that has mass and volume
Atoms
The fundamental building blocks of all matter.
Molecules
Are made of 2 or more atoms attached via chemical bonds.
The Scientific Method
Is a way of learning that emphasizes using to the senses produce knowledge through observations and experiments.
True or False: The Scientific method is a set of general principles that helps to describe how a scientist thinks.
True
Observation
Involve measuring or observing some aspect of nature.
Laws
Are summaries of the results of a large number of observations.
Hypotheses
Are tentative interpretations of the observations.
Experiments
Are highly controlled procedures designed to validate or invalidate hypotheses.
Theories
Are models that explain and give the underlying causes for observations and laws
Example) A blender doesn’t work when plugged in.
What scientific method is being used?
Observation
Example) The blender motor is broken.
What scientific method is being used?
Hypothesis
Example) The blender does not work when plugged into different outlets.
What scientific method is being used?
Experiment
Example) The blender needs repair.
What scientific method is being used?
Theory
Scientific Notation
Is a way of writing very large and very small numbers
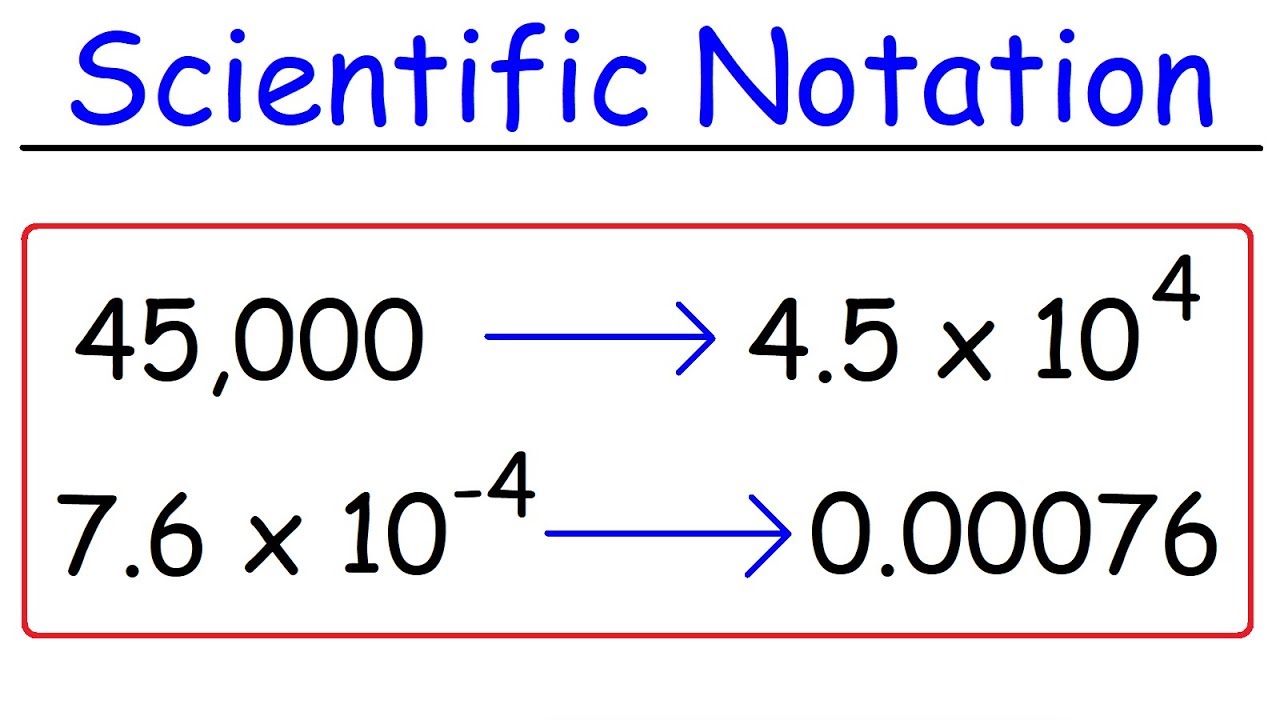
The number 98000 written in scientific notation would be?
9.8 × 104
The number 0.000008706 written in scientific notation would be?
8.706\cdot10^{-6}
Significant Figures
Is the system we use to write measurements.
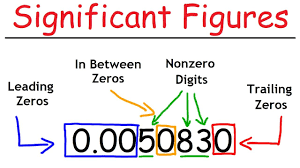
How many Significant Figures does the number 1.006 × 107 have?
4
How many Significant Figures does the number 23.070 have?
5
How many Significant Figures does the number 0.054901 have?
5
How many Significant Figures does 100 cm/m have?
Unlimited
How many Significant Figures does 10,000 kg have?
1
How many Significant Figures does 1 dozen = 12 have?
Unlimited
How many Significant Figures does the number 0.0035 have?
2
How many Significant Figures does 100 pens have?
Unlimited
How many Significant Figures does the number 120400 have?
4
How many Significant Figures does the number 130. have?
3
Accuracy
How close a measurement is to the accepted value.
Precision
How close a series of measurements are to each other.
16.84
Complete the operation: 5.74 + 0.823 + 2.6 =
Use the correct number of Significant Figures.
Answers:
A) 9.163
B) 9.160
C) 9.2
D) 9.1
C
Complete the operation: 1624 + 52.8 + 121.23 - 6.459 =
Use the correct number of Significant Figures.
Answers:
A) 1792
B) 1790
C) 1791.571
D) 1791.57
A
What is the answer for : 3.489 × ( 5.68 - 2.3 ) =
a) 11.79
b) 11.8
c) 12
d) 11.79282
C
tera-
Symbol: T
Meaning: trillion
(1012)
giga-
Symbol: G
Meaning: billion
(109)
mega-
Symbol: M
Meaning: million
(106)
kilo-
Symbol: k
Meaning: thousand
(103)
deci-
Symbol: d
Meaning: tenth
(10-1)
centi-
Symbol: c
Meaning: hundredth
(10-2)
milli-
Symbol: m
Meaning: thousandth
(10-3)
micro-
Symbol: μ
Meaning: millionth
(10-6)
nano-
Symbol: n
Meaning: billionth
(10-9)
Kelvin
Absolute scale
Celsius
Used in other countries
Fahrenheit
Used in the United States
Equation: Density
Density= Mass/Volume
Equation: Mass
Mass= Density x Volume
Equation: Volume
Volume= Mass/Density
Question: For a liquid substance with a density of 1.32 g/cm^3, what volume should be measured to deliver a mass of 68.4 g?
51.8\operatorname{cm}^3
Dimensional Analysis
Is a system that uses units as a guide to problem solving.
Question: Convert 25.3 in^3 to m^3? ( 1 in = 2.54 cm )^3 and ( 1 m = 100 cm )^3
A) 3.72 × 10^-4 m^3
B) 4.15 × 10^-4 m^3
C) 9.23 × 10^-4 m^3
D) 6.20 × 10-1 m^3
E) 1.87 × 10^-2 m^3
B
Question: A blood sample of 5.36 milliliters is collected from a patient to be analyzed for a platelet count. Human blood should have around 1.04 kg/L platelets. What is the expected mass in grams of platelets in the blood sample?
A) 6 g of platelets
B) 5.5744 g of platelets
C) 5.57 g of platelets
D) 5.574 g of platelets
C
Pure Element
Are the substances that cannot be broken down to simpler substances by chemical reactions.
Pure Compound
Are substances composed of 2 or more different types of atoms in fixed proportions.
Mixtures
Are composed of two or more different types of atoms that are not chemically bonded but simply physically mixed.
Homogeneous mixture
Is a mixture that has a uniform composition.
Heterogeneous mixture
Is a mixture that does not have a uniform composition.
Question: Classify the following as homogeneous or heterogeneous
Salt Water
Homogeneous
Question: Classify the following as homogeneous or heterogeneous
A mixture of table sugar and black pepper
Heterogeneous
Question: Classify the following as homogeneous or heterogeneous
Oil and vinegar salad dressing
Heterogeneous
Question: Classify the following as homogeneous or heterogeneous
Shampoo
Homogeneous
Question: Classify the following as homogeneous or heterogeneous
Peach pie
Heterogeneous
Compounds
Can only be separated into its components (elements) by chemical means (a chemical reaction).
Distillation
Separates components of a mixture according to their boiling points.
Filtration
Separates components of a mixture according to their physical state.
Question: Classify the following as pure substance or mixture
Air
Mixture
Question: Classify the following as pure substance or mixture
Helium
Pure substance
Question: Classify the following as pure substance or mixture
Blood
Mixture
Question: Classify the following as pure substance or mixture
Aluminum Foil
Pure substance
Question: Classify the following as pure substance or mixture
A 14 karat gold ring
Mixture
Solid
Atoms and molecules are packed close together and fixed in position.
Liquid
Atoms and molecules are closely packed, but they have some ability to move around.
Gas
Atoms and molecules are far apart from each other.
Properties of matter
1. Intensive Properties
2. Extensive Properties
3. Physical Property
4. Chemical Property
Intensive Properties
Independent of sample size.
( Ex: Density, Melting point, Boiling point, color, etc.)
Extensive Properties
Dependent of sample size.
(Ex: Mass, Volume, Energy, etc.)
Physical Property
Is a characteristic that a substance displays without changing its composition.
(Ex: Color, shape, taste, smell, m/b points, mass, electricity, malleability, and heat conduction.)
Chemical Property
Is a characteristic that a substance displays only through changing its composition.
(Ex: Acidity, basicity, oxidation (rusting of iron), combustion (burning coal).
Physical change
Are changes that alter the state or appearances of matter without altering its composition.
1. Change in the state of matter (solid, liquid, gas).
2. Change in the physical shape.
(Ex: Boiling water, sugar dissolving in coffee, seawater evaporating).
Chemical change
Are those changes that alter the composition of matter.
1. Results in a new composition.
2. Results in a new chemical and physical properties.
(Ex: Burning oxygen, rusting of iron)
Question: Classify the following change as physical or chemical
An egg splitting open and spilling out
Physical
Question: Classify the following change as physical or chemical
Sugar fermenting
Chemical
Question: Classify the following change as physical or chemical
Bubbles escaping from soda
Physical
Question: Classify the following change as physical or chemical
Bubbles that form when hydrogen peroxide is mixed with blood
Chemical
Question: Classify the following change as physical or chemical
Evaporation of rubbing alcohol
Physical
Question: Classify the following change as physical or chemical
Sugar turning black when heated
Chemical
Energy
Is the capacity to do work or transfer heat.
Kinetic Energy
Is the energy associated with motion.
(Ex: Swimming, Working out, Water flowing over a dam).
Potential Energy
Is the energy that is stored energy due to position of an object or its composition.
(Ex: A compressed spring, chemical bonds in food, water at the top of a dam)
Question: Identify the energy as potential or kinetic
Gasoline in the gas tank
Potential
Question: Identify the energy as potential or kinetic
A peanut butter and jelly sandwich
Potential
Question: Identify the energy as potential or kinetic
Mowing the lawn
Kinetic
Temperature
Is a measure of the thermal energy of a substance (not the exchange of thermal energy.)
Law of Conservation of Matter
Matter is neither created nor destroyed in a chemical reaction.
Law of Conservation of Energy
Energy is neither created nor destroyed by can change from from one form to another.
Exothermic
Are those reactions that result in the release of Energy.
Reactants have less potential energy than products
Exothermic=heat
Endothermic
Are those reactions that result in the absorption of Energy.
Products have more potential energy than reactants.
Endothermic=cold