10.1-10.2/ OZ 4- Rates of Reaction
1/99
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
100 Terms
Define rate of reaction
· The rate at which reactants are converted into products
· Change in concentration of a reactant or a product in a given time
· The rate at which a quantity changes
What is the rate of product formation equal to, give an example of this?
The rate at which the reactants are used up
Decomposition of hydrogen peroxide (H2O2)- the rate of reaction is the rate at which H2O(l) and O2(g) are formed which is the same as the rate at which H2O2 (aq) is used up
2H2O2 (aq) --> H2O (l) + O2 (g)
Rate can be measured in moles of water or oxygen formed per second or moles of hydrogen peroxide used up per second
Give some examples of some fast chemical reactions
Combustion/ precipitating of silver halides
Give some examples of some slow chemical reactions
Souring milk, rusting, diamond to graphite (millions of years)
What is happening to ozone all the time?
It is made and formed?
What is a steady state of a reaction?
If left to themselves these reactions would reach a point where e.g. ozone was being made as fast as it was being used up
Why di chemists study the rate of reaction/ reaction kinetics?
Helps chemists to find ways of controlling and predicting chemical reactions
What should be measured to calculate the rate of reaction?
A property that changes during the reaction and that is proportional to the concentration of a reactant or product
What is the key equation to calculate the rate of any reaction, what is the units?

What is the rate of reaction equal to on a graph, how do you calculate this?
Gradient of the graph, draw a tangent- change in y/ change in x
Draw a diagram showing products and reactions concentrations during a reaction against time
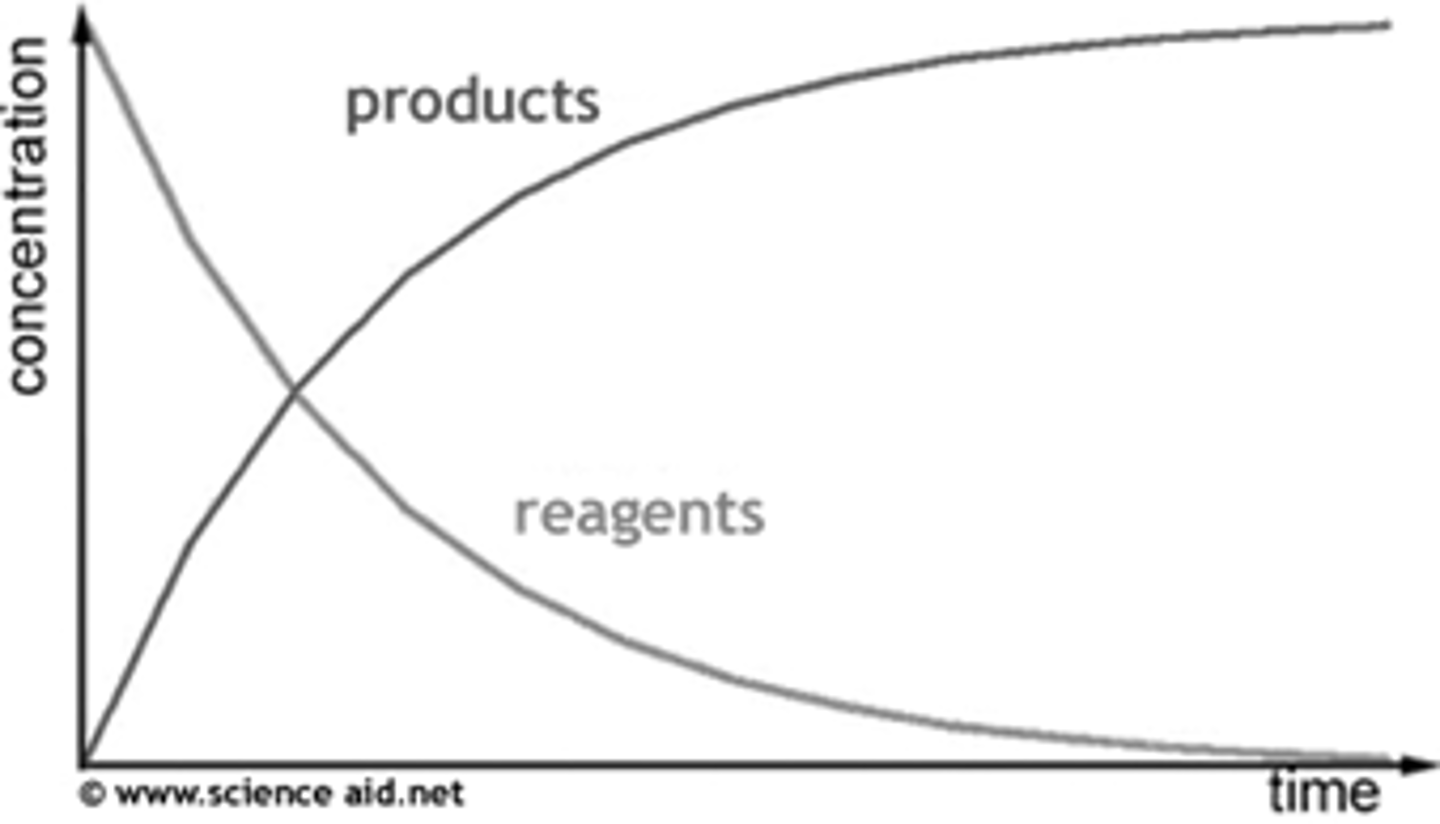
When in a reaction is the rate the fastest?
Initial rate (at the start of the reaction) is always the fastest- highest concentration of the reactants, more frequent collisions so fastest rate
What happens to the rate of reaction over the course of a reaction?
Over the course of the reaction reactants are used up (as they form products) and the concentration decreases, less frequent collisions between reactants and the rate of reaction slows
When is the rate of reaction the slowest?
Final rate at the end is the slowest - levels off when the gradient is zero the reaction is finished
When does the rate become 0 and the reaction stops?
When one of the reactants has been used up the rate becomes zero- the reaction stops
What two different types of rate measurement are there?
Can measure the loss of a reactants or the formation of a product at regular intervals throughout a reaction
What are the 5 methods used to calculate the rate of a reaction, which method is best for which reaction?
1. Measuring the volume of gas produced- if one of the products is gaseous
2. Measuring the loss of mass if a gas is produced
3. Measuring the change in pH during a reaction
4. Measuring a change in temperature if it is exo/ endothermic
5. Taking samples at regular intervals and analysing them by titration
Give the flow diagram to show how you decide which method of rate measurement to use
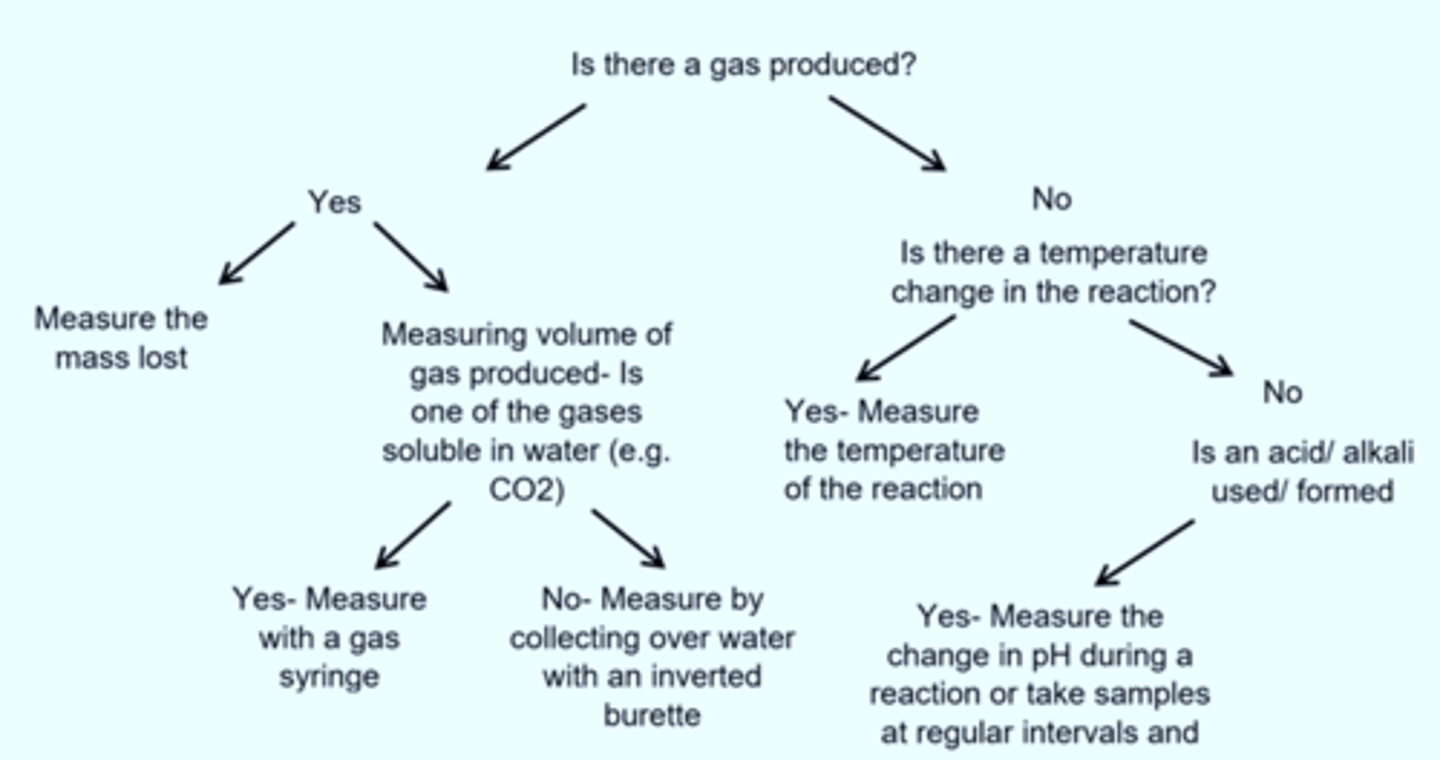
What should you do with the data collected for the rate?
· Plot a graph and follow the course of a reaction
· Time is plotted on the x-axis and the change you measure goes on the y-axis
· State if the rate of decomposition is directly proportional to the concentration
How do you find the rate of reaction from a graph?
Draw a tangent to the curve at the time, the gradient of tangent is the rate of reaction
How do you improve the experimental predicted rate?
Test more concentrations/temperatures to plot more points on the graph and make it more accurate and confirm a relationship
What is one of the most likely cause of anomalies for reactions involving solutions?
Mistake diluting the solution, make fresh e.g. H2O2 solutions for each one
When should you calculate rate by measuring the gas evolved?
In any reaction where a gas is produced
Name the two types of method to measure the gas produced to calculate the rate?
Measuring the gas produced
Measuring the mass lost
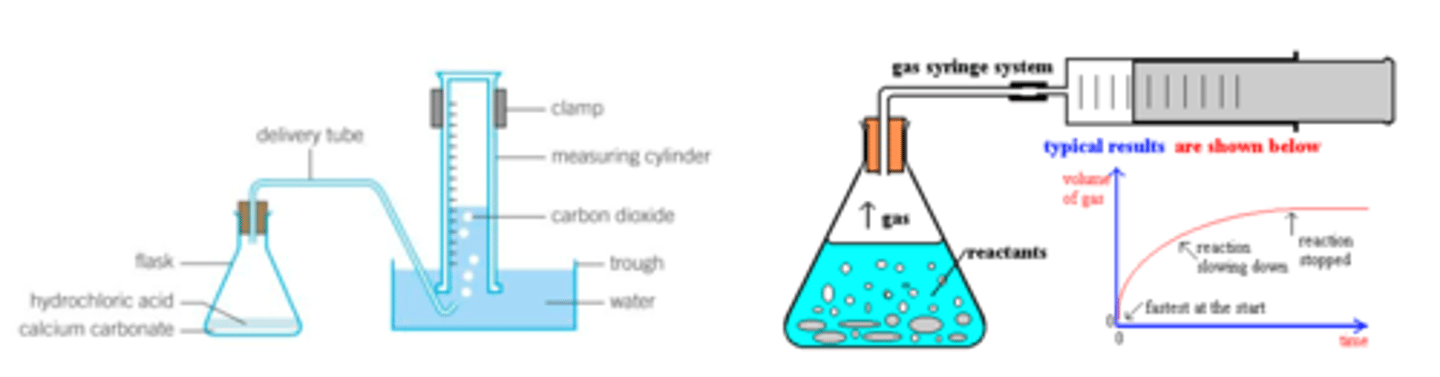
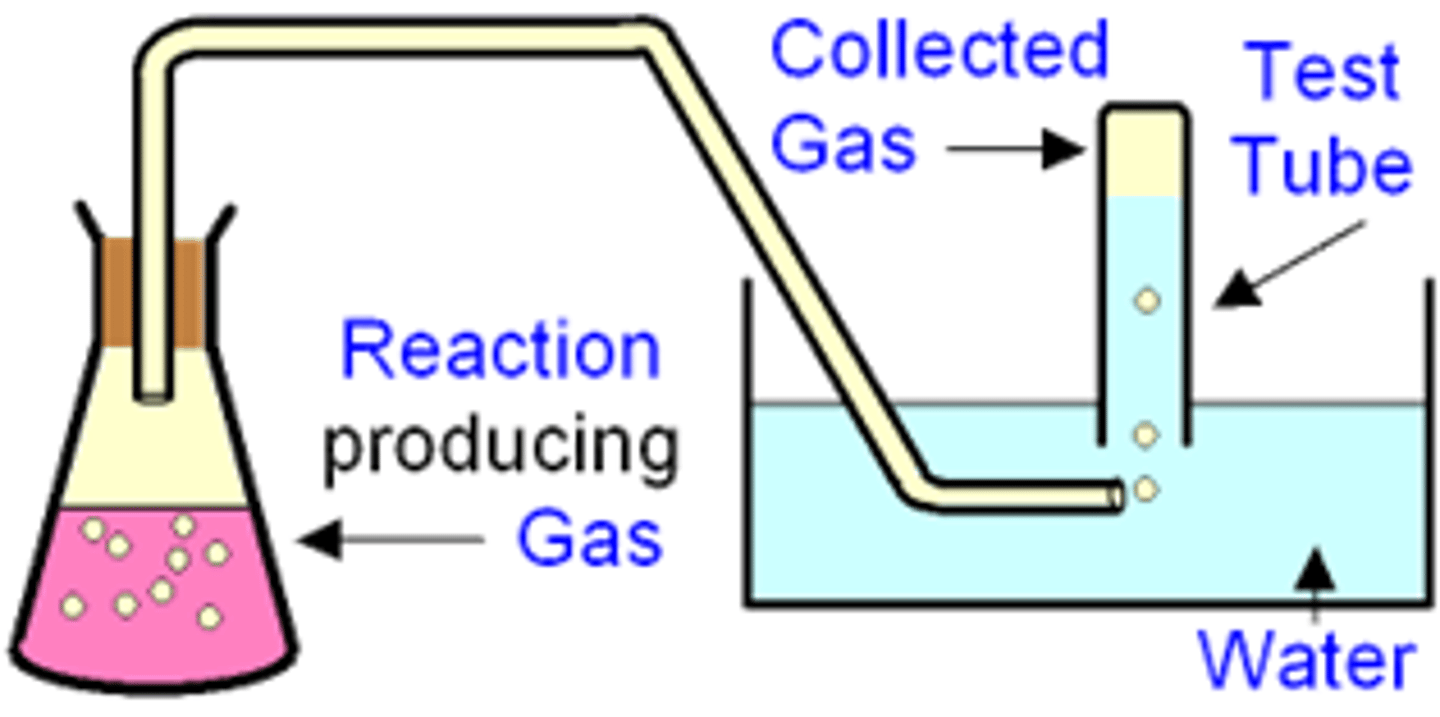
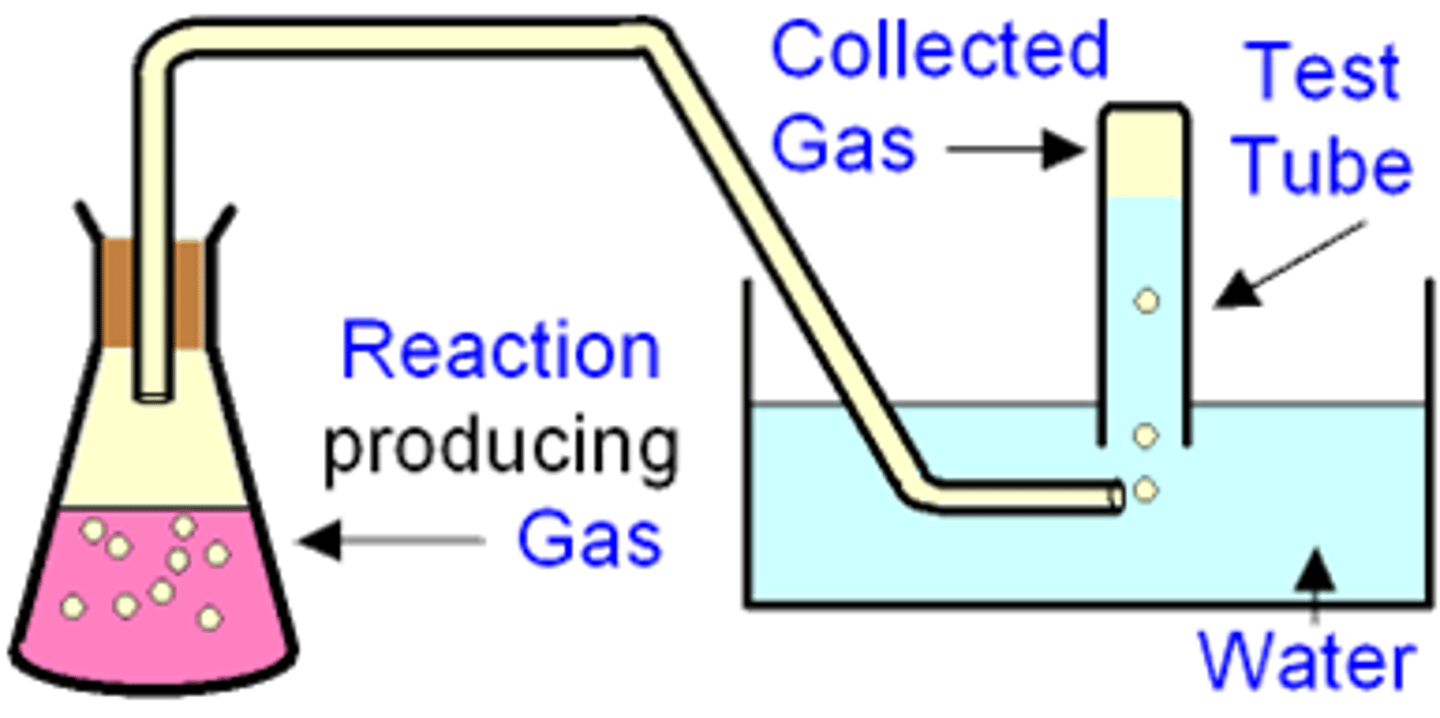
What are the two methods of collecting the gas produced from a reaction, when should you do each, draw diagrams for each?
1. Collect over water in an inverted burette- gas displaces the water
1. Collect in a gas syringe- do this if the gas produced is soluble in water
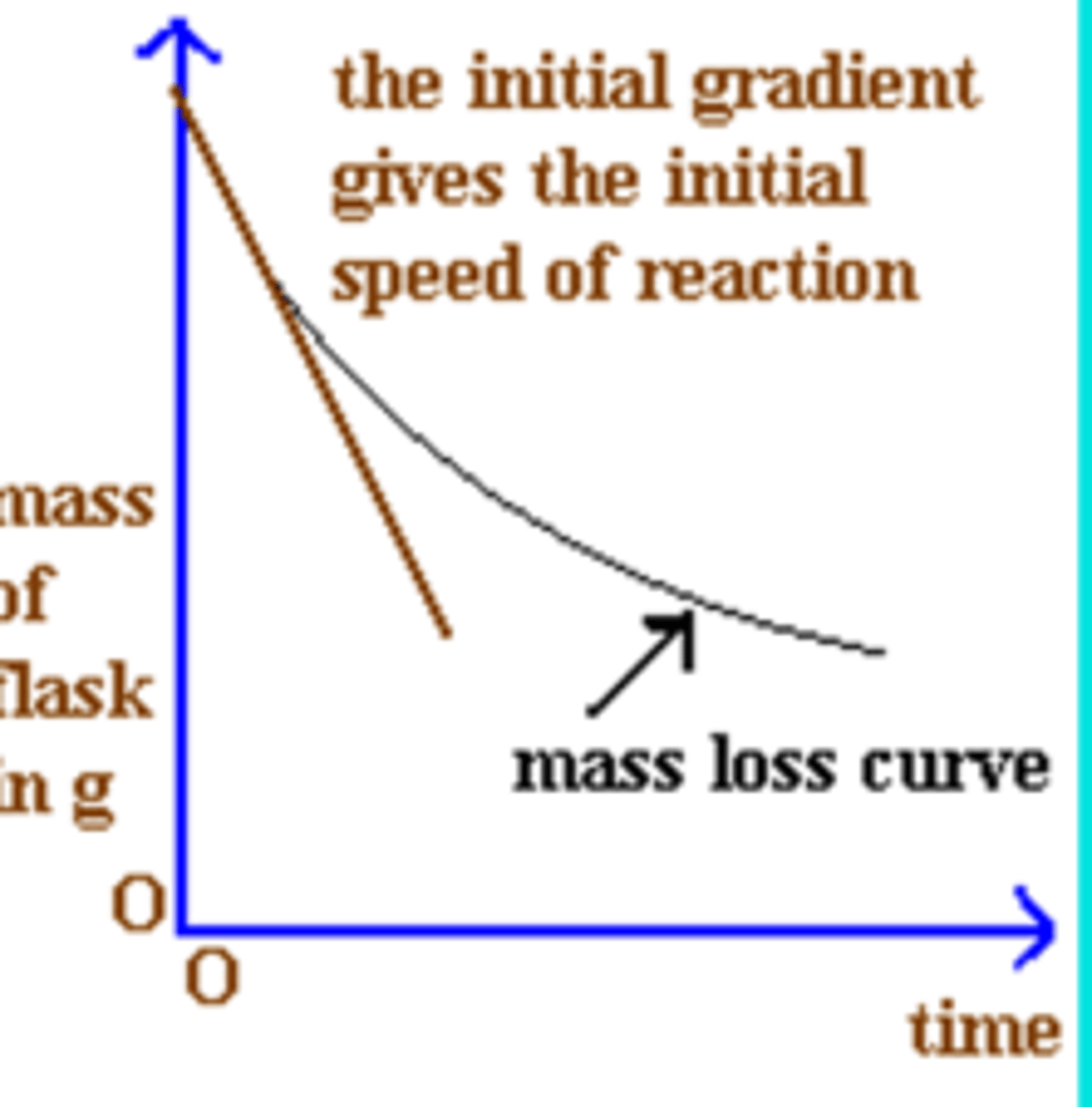
Draw a diagram for a method to measure the mass lost when gas is produced in a reaction, draw the expected graph and how you would calculate the rate, what are the units?
Change in mass- g/s
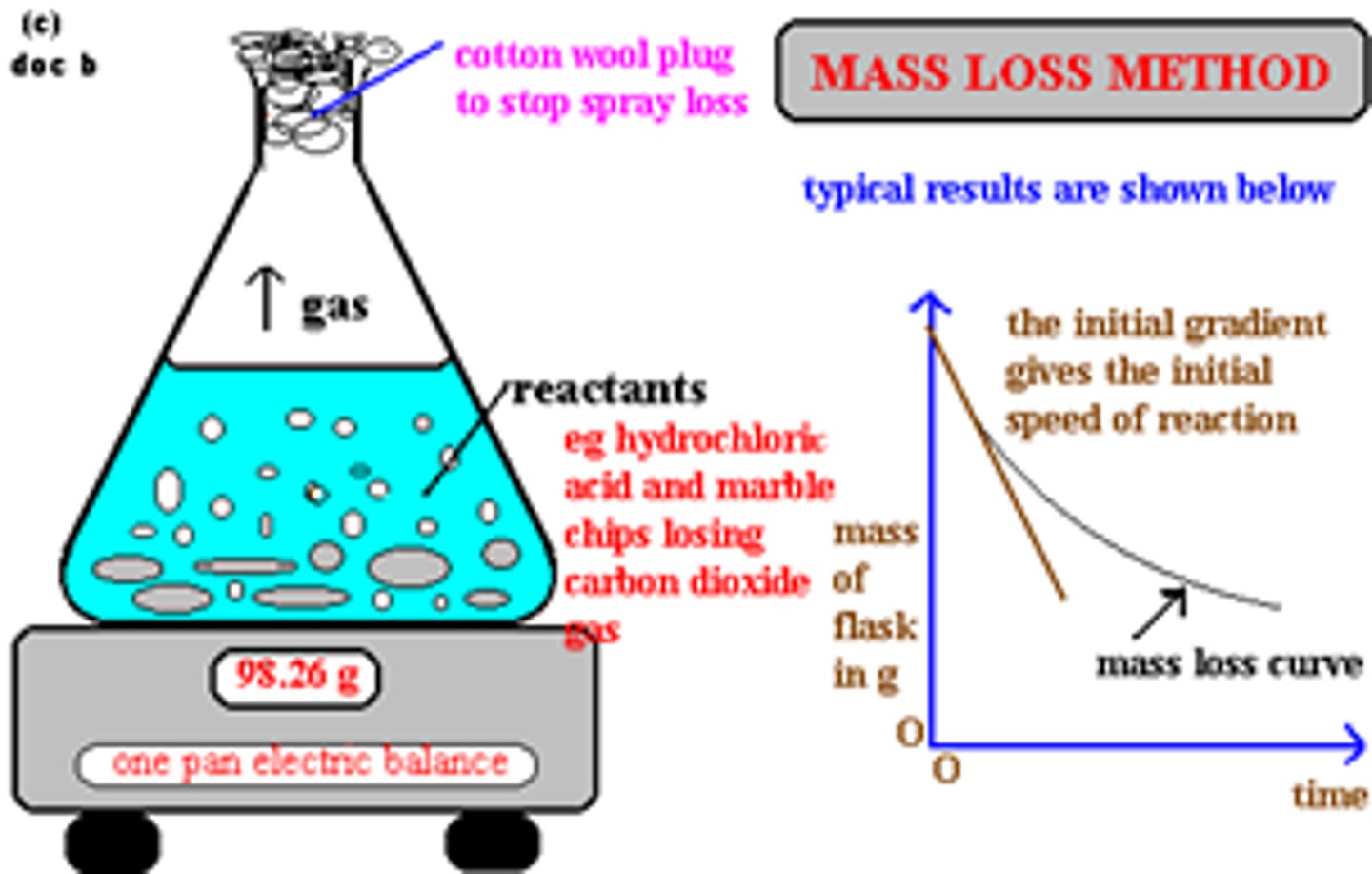
Why would you do an experiment to calculate the mass lost when gas is produced rather than collecting the gas?
One of the gases produced is soluble in water so collecting over water, it will dissolve and the full amount of gas produced will not be collected
Give an example of an experiment of where you would collect the gas produced to calculate the rate?
Mass of gas lost- Calcium carbonate and hydrochloric acid produce carbon dioxide which can be collected
· The volume produced can be used to follow the rate of reaction
Volume of gas collected- Could also be used for the decomposition of hydrogen peroxide
When measuring the rate of reaction by collecting the gas produced what sort of graph should you draw?
Graph of volume against time indicates rate
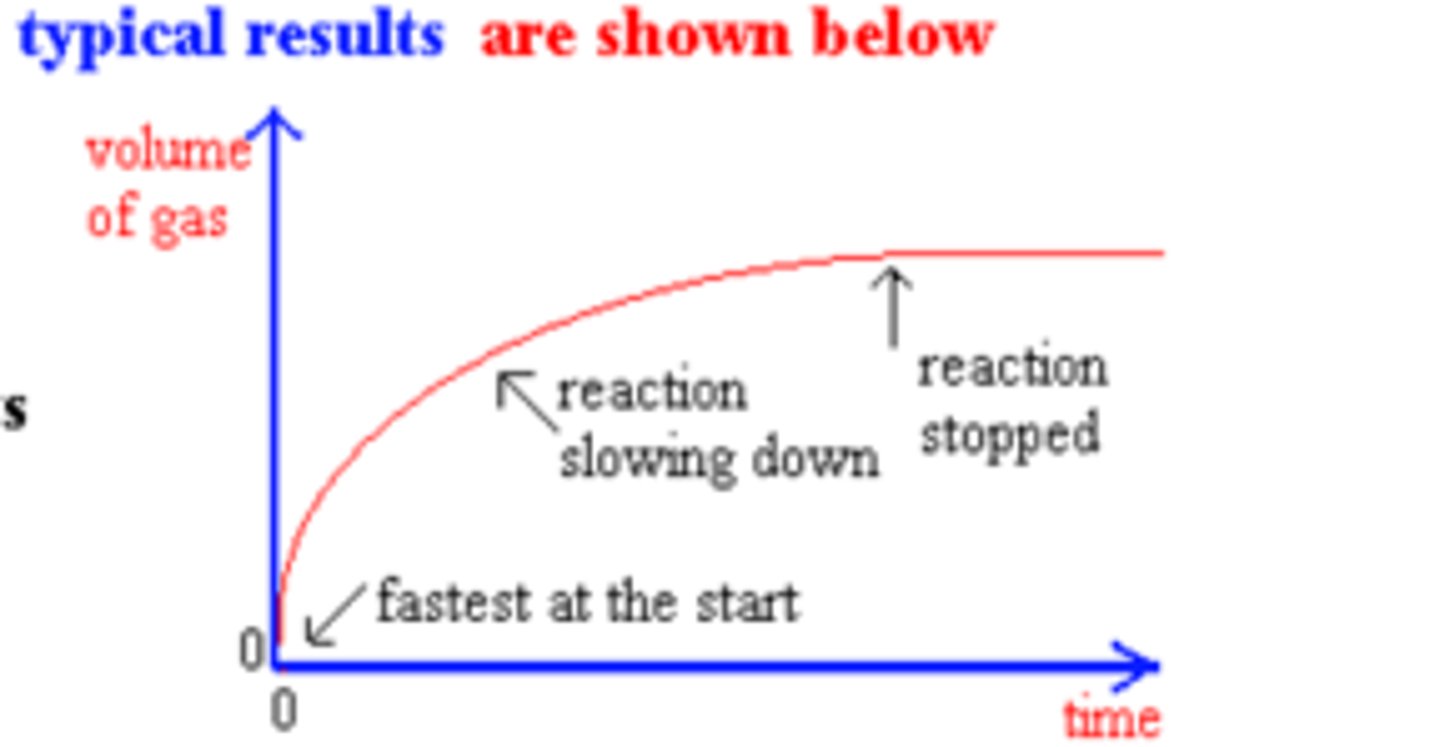
When measuring the rate of reaction by measuring the mass of gas lost what sort of graph should you draw?
Mass of flask/ beaker against time
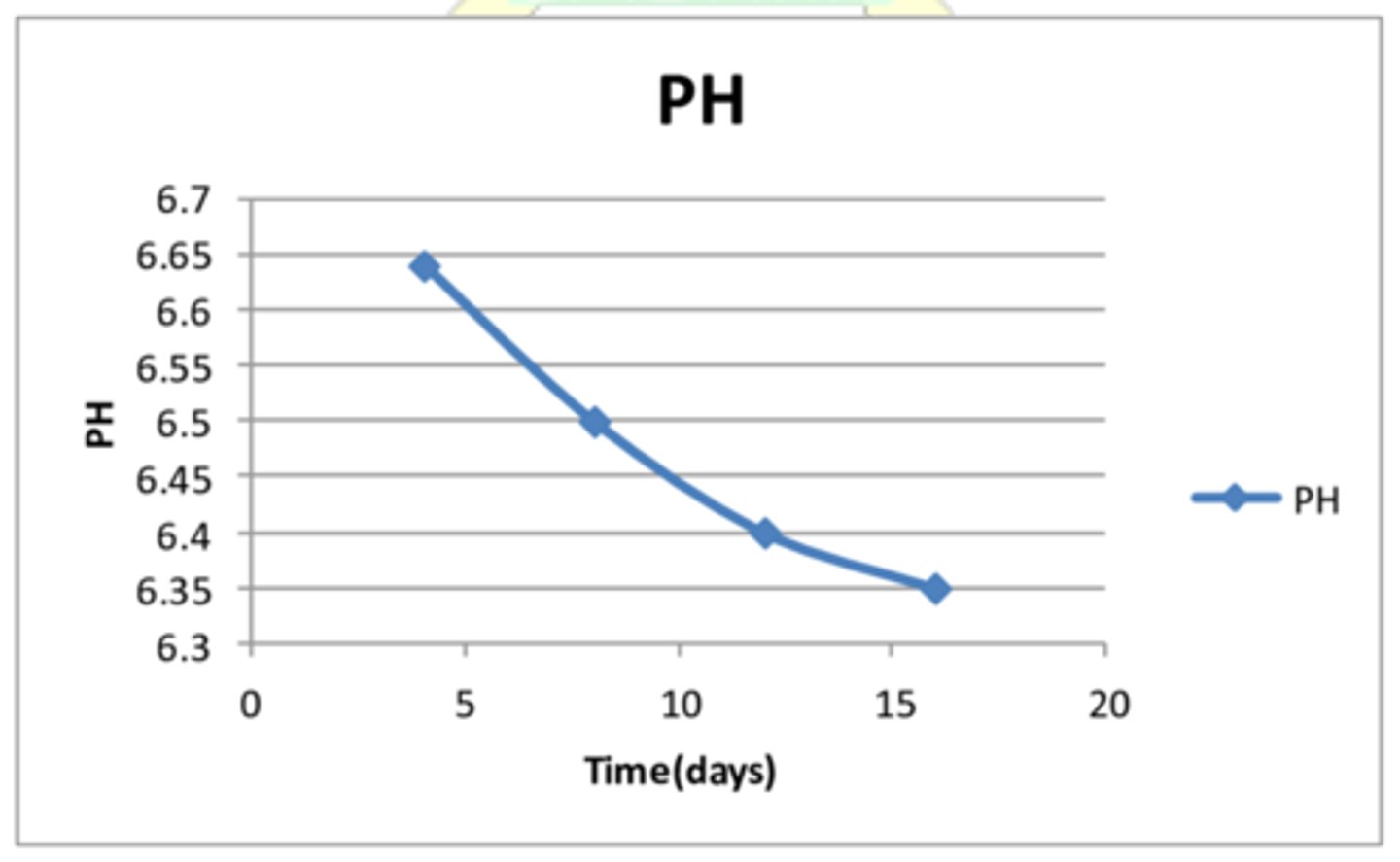
How can measuring the pH of the reaction mixture help to calculate rate, give an example of a reaction this could be used for and an example graph?
Measuring the pH of the reaction mixture can be used to measure rate as an acid/ alkali is used up/ produced
EXAMPLE- Reaction between HCl and calcium carbonate- as the reaction proceeds the hydrochloric acid concentration will fall as it reacts with the calcium carbonate so the pH will increase
Unit for this?
How can measuring the colour changes of the reaction mixture be used to calculate the rate, give and example of a reaction with this could be done for?
The colour of a reaction mixture can change as certain products/ reactants are used up/ produced
Colorimeter- measures the change in colour of a reaction
EXAMPLE- Zinc and aqueous copper (II) sulphate. Blue colour of the copper sulphate solution decreases and the reaction can be followed using a colourimeter.
Zn(s) + CuSO4 (aq) ZnSO4(aq) + Cu(s)
Unit for this?
What is different about using chemical analysis to measure the rate?
It interferes with the progress of the reaction, the other techniques do not
How can chemical analysis be used to measure the rate of reaction, what is the technical word used for this?
Chemical analysis- take samples of the reaction mixture at regular intervals and stopping the reaction in the sample (quenching it) before it is analysed
Give an example of a reaction where you would use chemical analysis to calculate the rate?
Iodine and propanone- react in the presence of an acid catalyst- a sample can be extracted from the reaction mixture and quenched by adding sodium hydrogen carbonate to neutralise the acid catalyst stopping the reaction, then determine the amount of unreacted iodine remaining by titration
Could plot e.g. concentration of acid against time
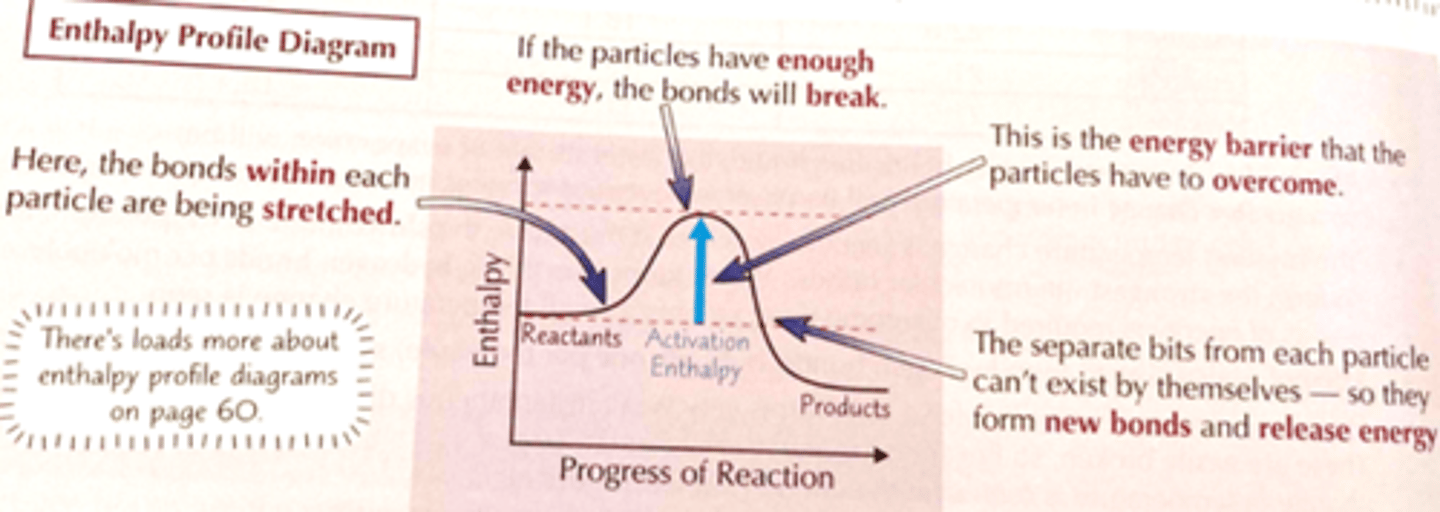
What does an enthalpy diagram show, draw one?
The reaction progress against time
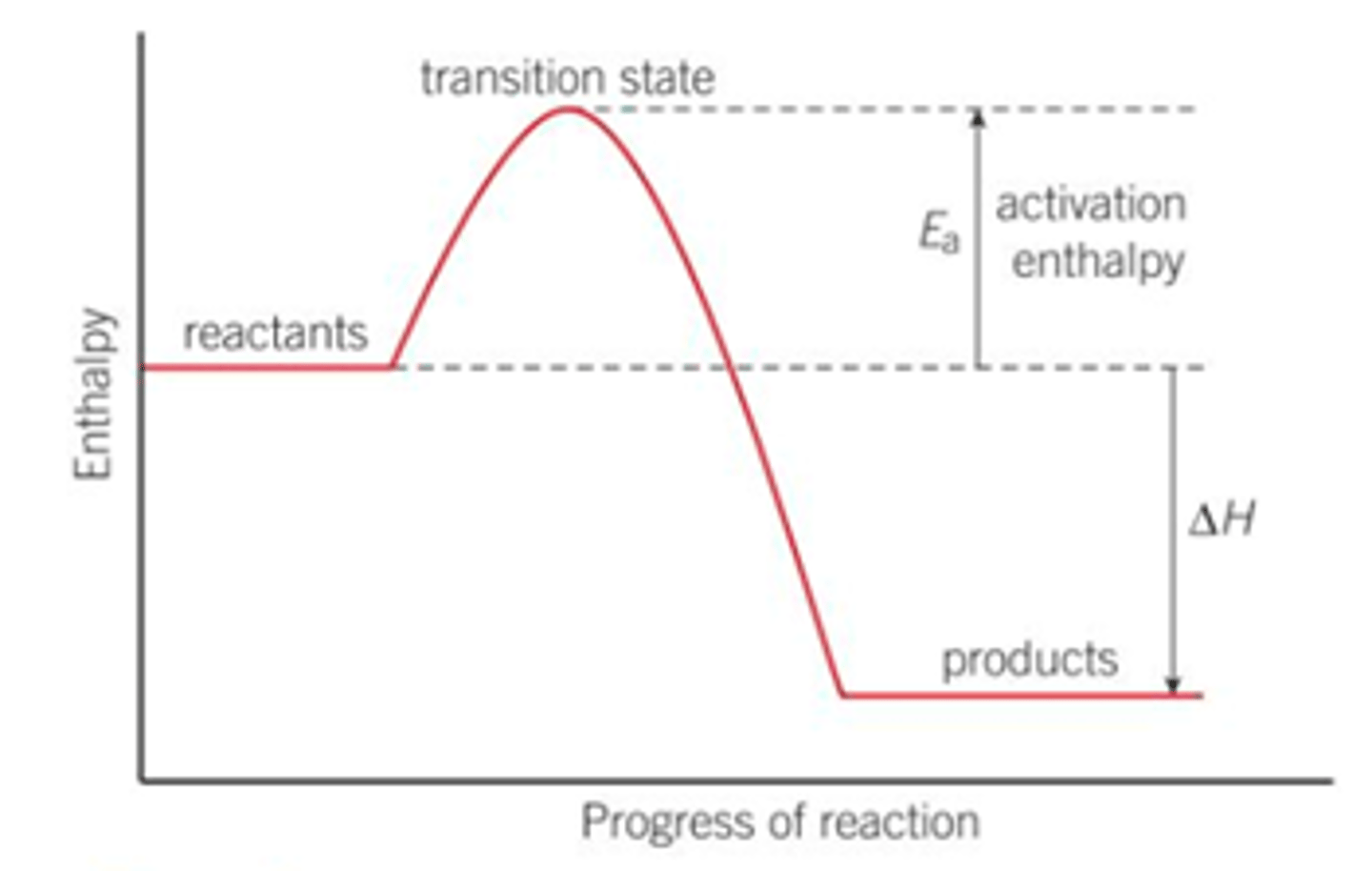
What is the highest point on the reaction pathway of an enthalpy profile diagram?
The highest point on the pathway corresponds to the transition state where old bonds stretch and new bonds start to form- this state exists for only a very short amount of time
What does only one change in an enthalpy diagram indicate, what does multiple curves mean?
This reaction above is a simple reaction with one step
Many reactions take place in a series of steps and there will be many curves- one for each step
What happens when particles approach and collide
Existing bonds start to stretch and break, new bonds form
Annotate and enthalpy profile diagram to show where the bonds stretch, break and new bonds form
What are the 6 key factors which can affect the rate of reaction, explain how?
1. Concentration- of reactants, higher concentration reactants- faster the reaction, solution- concentration measures in moldm-3, in gases concentration is proportional to the pressure
2. Pressure- when the reactants are gases, same effect as concentration
3. Temperature- nearly all reactions go faster at higher temperatures
4. Intensity of the radiation- if the reaction involves radiation increased intensity- faster the reaction, dissociation of oxygen molecules happens faster when the intensity of the radiation increases
5. Particle size- of a solid, reacts faster when finely powdered, powder reacts faster than lumps of solid as there is a much larger surface area of the solid exposed for the reaction to take place on (a more finely divided catalyst can also affect the rate)
6. Presence of a catalyst
What is reaction kinetics?
The study of the factors that alter the rate of a chemical reaction
If there is a large spread in experiment data results what should you do?
If there is a large spread the data in an experiment repeat the data set
What is the general unit of rate for any reaction?
change (concentration/ mass etc.)/ unit of time or unit time^-1
Define Collision Theory
Reactions occur when the particles (atoms, ions or molecules) of reactants collide, provided they collide with a certain minimum kinetic energy. This is called the activation energy.
Define activation energy
Minimum amount of kinetic energy needed by colliding particles to allow them to react is called activation enthalpy or activation energy the particles need this much energy to break the bonds to start the reaction
Which reactions happen easily and which require heating?
For reactions with low activation enthalpy they happen easily, for reactions with high activation enthalpy they must be heated to give the particles more energy
Does every collision cause a reaction?
Not every collision causes a reaction- two key conditions must be met for a reaction to occur (activation energy and orientation)
What two conditions must be met for two particles which collide to react?
Particles in liquids and gases are always moving and colliding with each other, but they only react when the condition are right- the activation energy and the orientation
CONDITION 1- Describe the activation energy that the particles need in order to react, draw a diagram?
· They must collide with at least a certain minimum amount of kinetic (movement) energy equal to or greater than the activation energy of the reaction allowing a reaction to occur and go on to produce products
· The molecules must have sufficient energy to overcome the activation energy of the reaction
· Combined energy of the two particles on collision
· e.g. ozone molecule and chlorine radical must collide and must have enough KE when they collide to react
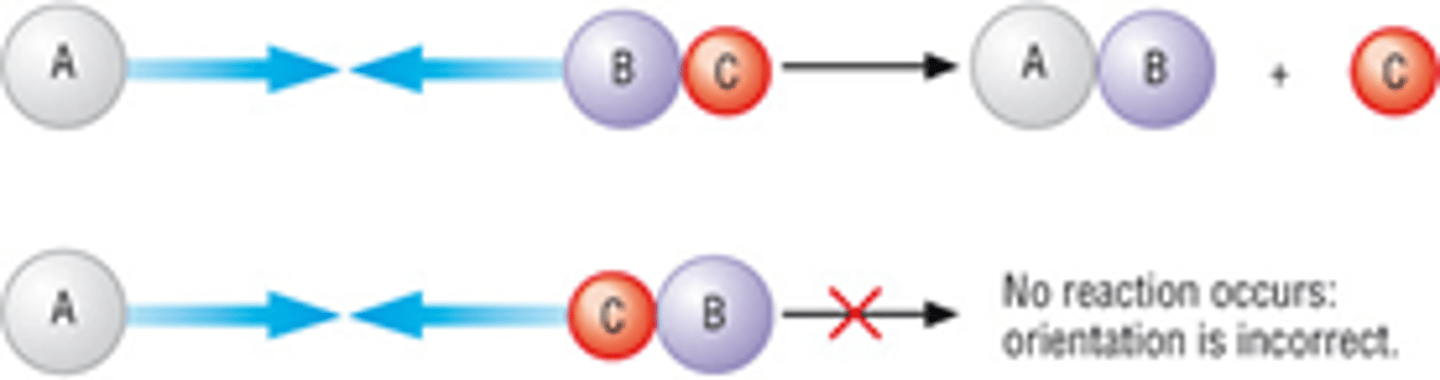
CONDITION 2- Describe the orientation that the particles need in order to react, draw a diagram?
· The molecules must also collide with the correct orientation for the reaction to take place, even if they have sufficient energy
· Collide in the right direction- need to be facing each other the right way- head on
· E.g. A + BC --> AB + C
Must collide with B for a reaction to take place
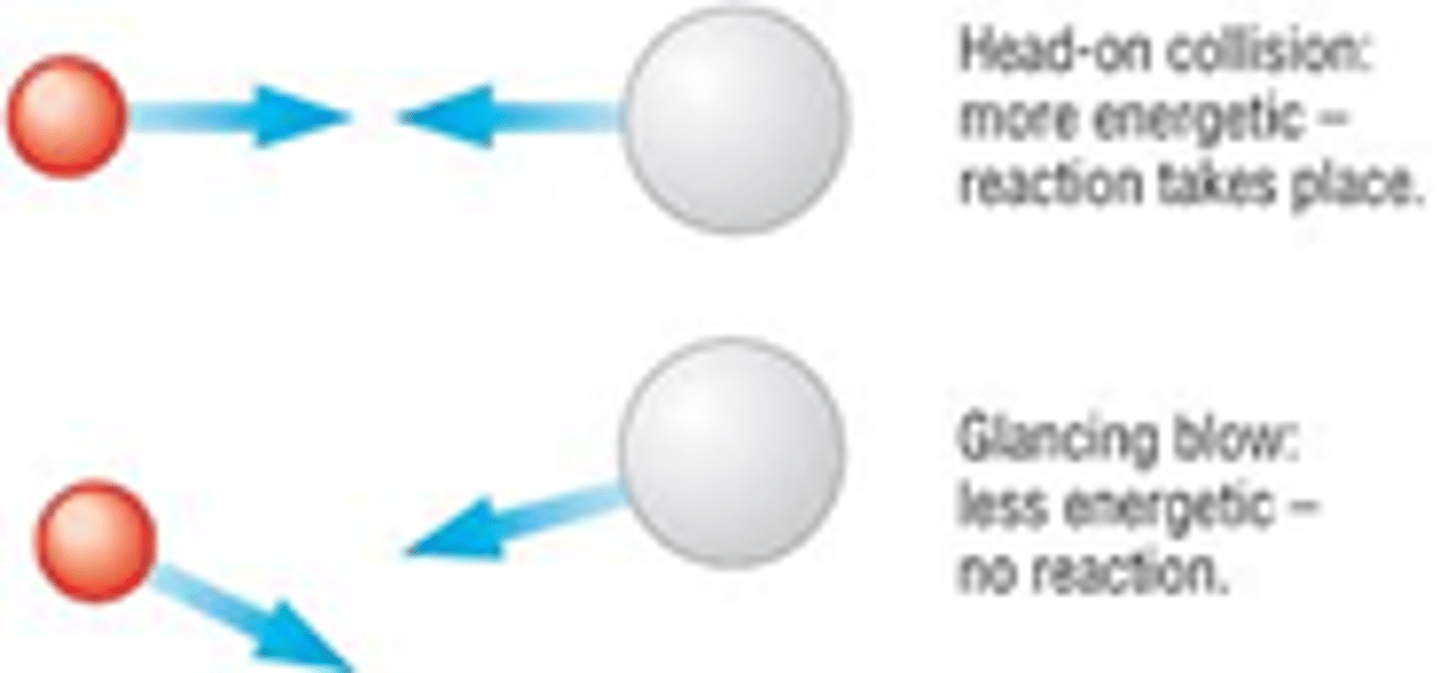
· Head on collision- more energy energetic, reaction takes place
· Glancing blow- less energetic, no reaction
Which factors decide the rate of reaction?
1. Frequency of collisions- Number of molecules in the system/ say how close they are instead
2. Their energy- what proportion are able to overcome activation energy when they collide these are the two factors which affect the rate of reaction
When answering a question about the effect of changing a condition on the rate of the reaction how should you answer it?
Must state and explain the effect of changing the conditions on these two factors (energy and frequency of collisions) in an answer
Use collision theory in answers
What does a higher concentration do to the rate of reaction?
High concentration of reactants (in solution) lead to a greater rate of reaction
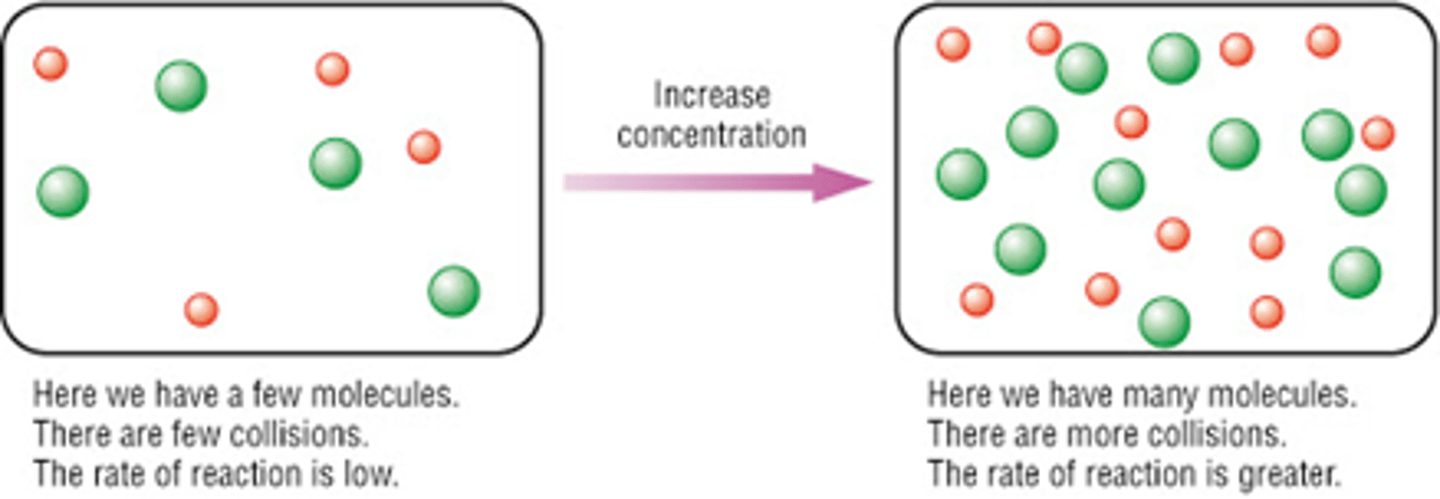
Describe in 5 steps according to collision theory how concentration affects the rate of reaction, draw a diagram?
1. Higher concentrations (and pressures)- means more molecules in the same volume so the particles/molecules are in closer proximity to each other on average
2. So they collide more frequently/ greater chance of the molecules colliding/ more collisions will occur in a certain length of time
3. There are more collisions per second- higher frequency of collisions.
4. More collisions per second means more chance to react
5. The rate of reaction increases
EXAM TIP- Should you say more molecules in the same area or volume for increased concentration?
Increases concentration say more molecules in the same VOLUME (of solution) not area
EXAM TIP- What must you say instead of "more collisions"
You cannot just say “more collisions” you must refer to a time frame “more collisions per second” so instead use the phrase a HIGHER FREQUENCY OF COLLISIONS
What happens to particles when you increase the pressure/ concentration?
There are the same NUMBER of molecules just in a smaller space so closer together- more frequent collisions
Describe an experiment to investigate the effect of concentration on the rate of reaction, draw a diagram?
· Use a burette or measuring cylinder
· Continuous monitoring of the volume of gas
· Plot the volume of gas against time
· Find the initial rate of reaction (tangent at t = 0)
· Repeat the experiment with a different concentration of acid keeping everything else constant
· To create different concentrations of acid use varying concentrations of acid and water each time with the same total volume of liquid
Use another burette to measure the volume of water
When should you talk about concentration and when should you talk about pressure?
They have the same explanations for collision theory
Liquid/ solution- concentration
Gas- pressure
What does a higher pressure do to the rate of reaction?
A higher pressure of gas reactants increases the rate of reaction
What happens to the particles when the pressure is increased?
Molecules are pushed closer together on average. The same number of molecules occupy a smaller volume
If not all the reactants/ products are in a gaseous state does pressure still affect rate?
Yes?
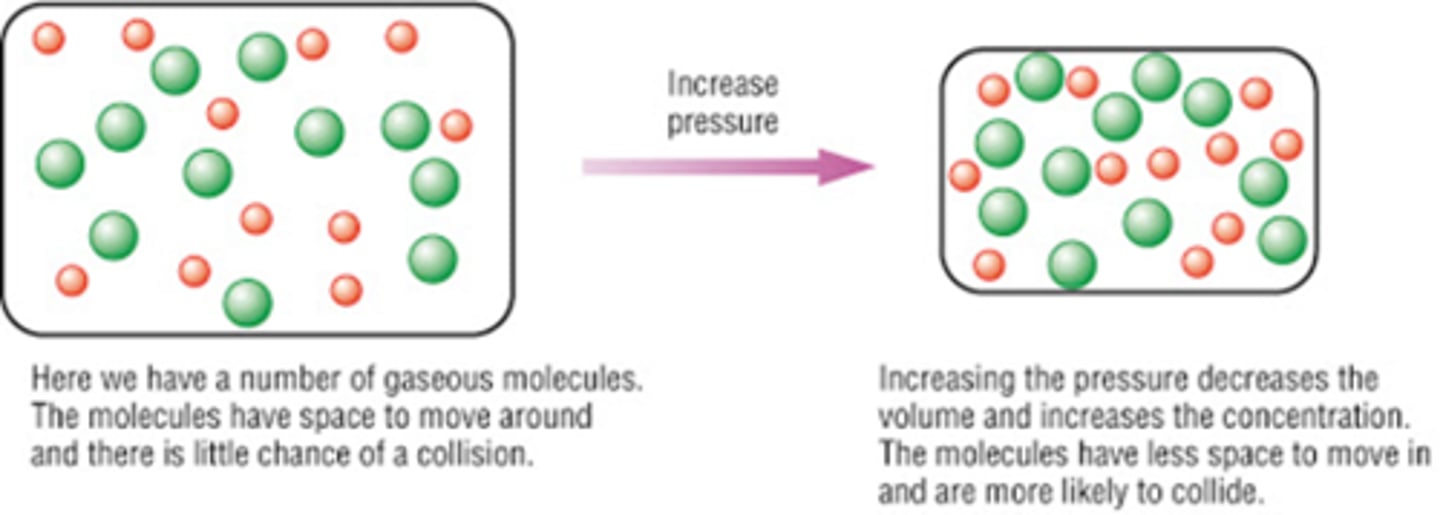
Describe using collision theory how a higher pressure can affect the rate of reaction, draw a diagram?
1. Higher pressures- means more molecules in the same volume so the particles/molecules are in closer proximity to each other on average
2. So they collide more frequently/ greater chance of the molecules colliding/ more collisions will occur in a certain length of time
3. There are more collisions per second- higher frequency of collisions.
4. More collisions per second means more chance to react
5. The rate of reaction increases
(Same explanation as a higher concentration)
Why are reactions very slow in solids?
When particles are in fixed positions in the number of collisions is low
What is the effect of smaller particle sizes on the rate of reaction?
Smaller particles lead to an increased rate of reaction
Describe using collision theory how a smaller particle size can affect the rate of reaction?
1. Smaller particles have a larger surface area- Larger surface area provides greater chance of successful collisions
2. So there is a greater surface area for collisions with other reactant particles
3. So there is a higher frequency of collisions
4. This leads to a greater rate of reaction
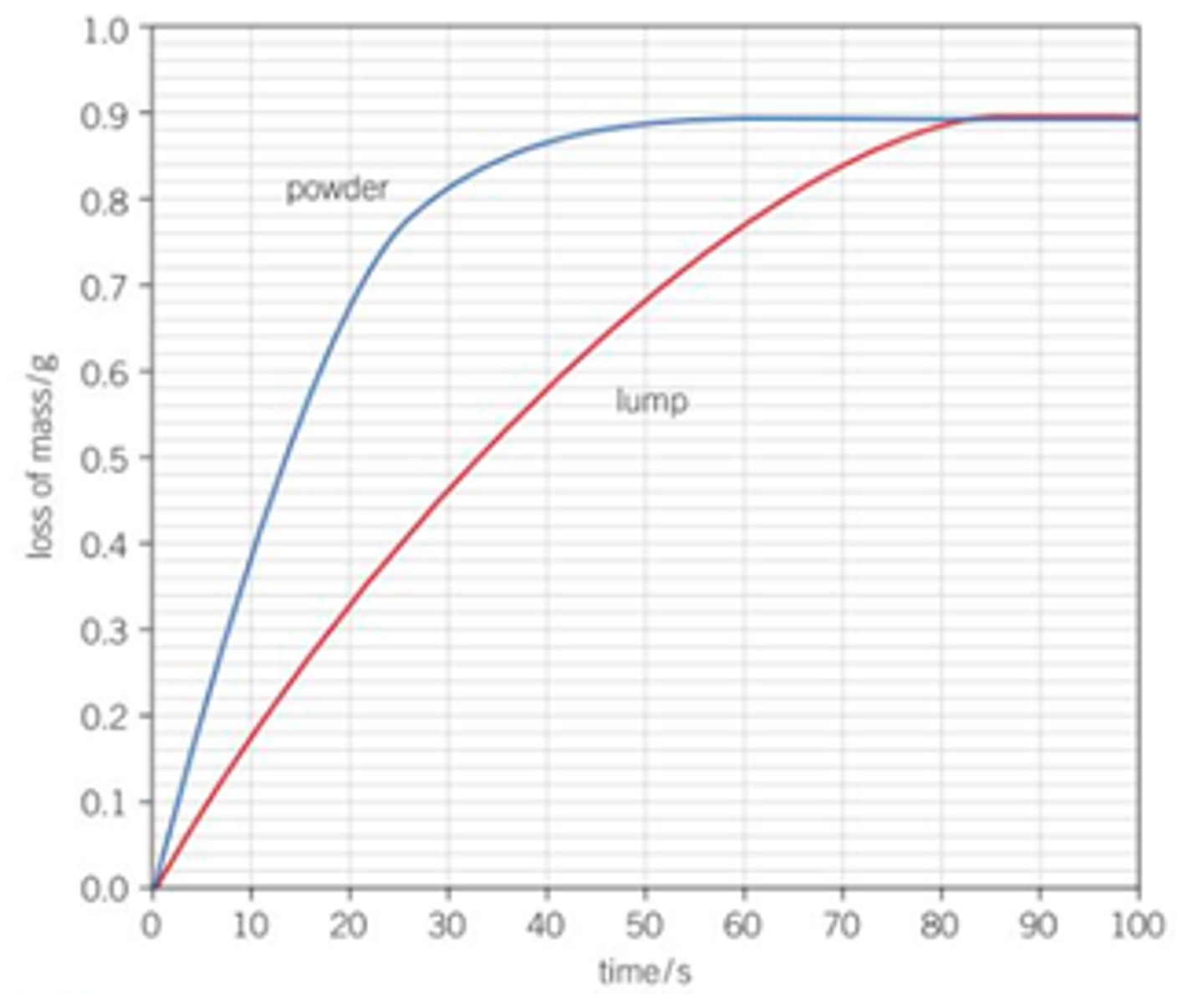
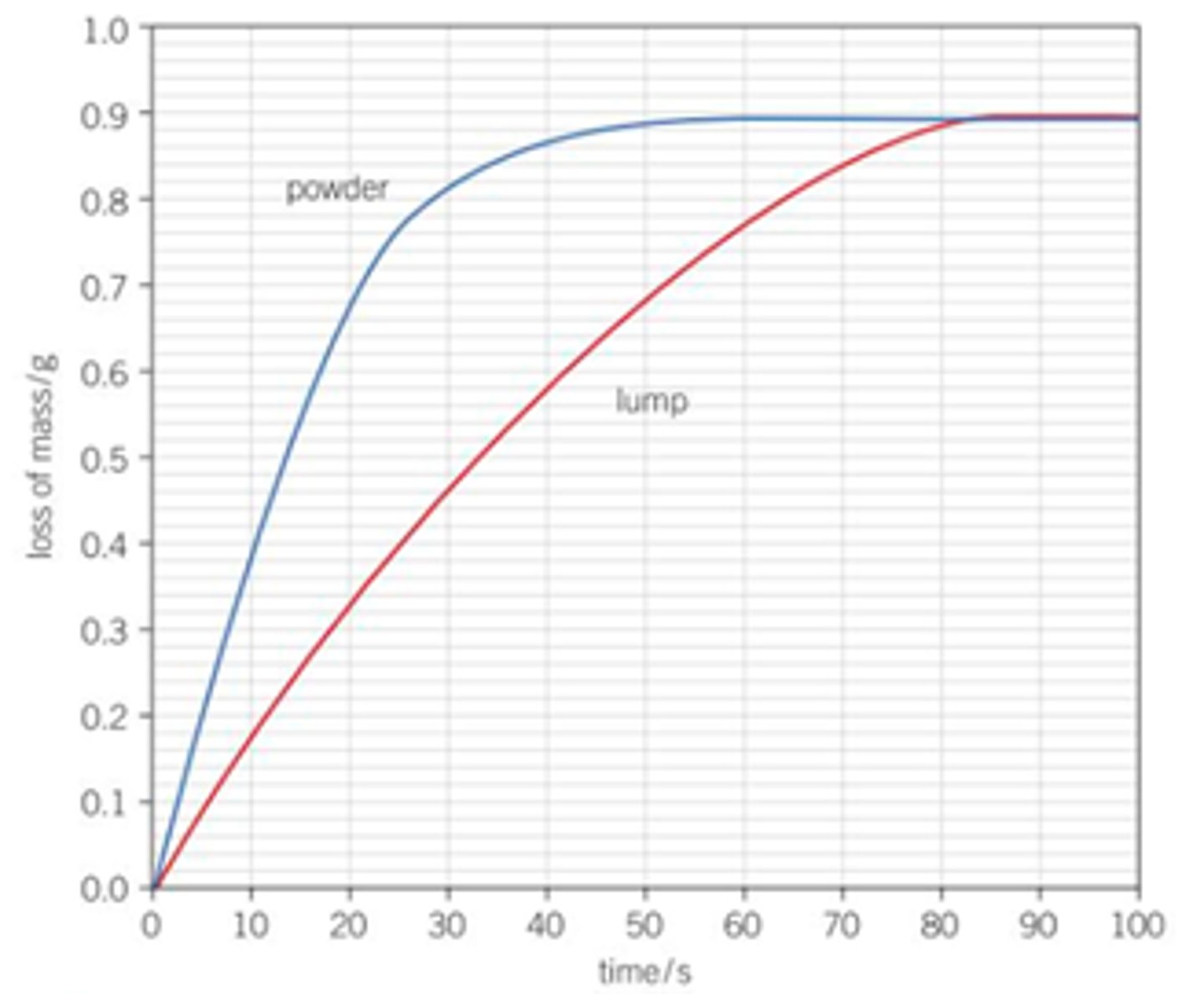
Draw a graph for smaller particle size and rate of reaction, how can you see from the graph that the rate is higher with a smaller particle size?
Steeper gradient for the powder and it levels off sooner- faster rate and the reaction finishes sooner
For most reactions what is the effect of a 10 degree C/K rise in temperature on the rate?
Roughly doubles the rate
Explain using collision theory the two reasons that a higher temperature leads to a faster rate of reaction
1. At a higher temperature the particles have more kinetic energy, so they are moving faster so they collide more frequently (increased frequency of collisions) so there is a greater rate of reaction
2. At a higher temperature the average energy of the particles is higher so a greater proportion of the particles/ colliding pairs have energy greater than or equal to the activation energy when they collide, so a greater proportion of the collisions are successful and allow the particles to react
What can be used to easily overcome activation energy and cause a reaction?
Sparks
Describe an experiment used to show how the temperature affects the rate of reaction, draw a diagram?
· Use a burette or measuring cylinder
· Continuous monitoring of the volume of gas
· Plot the volume of gas against time
· Find the initial rate of reaction (tangent at t = 0)
· Repeat the experiment at a different temperature keeping everything else constant- put the boiling tube/ conical flash in a beaker/ water bath and use hot water from a kettle/ cool water or an electric water bath to change the temperature
· Measure the temperature of the solution not the temperature of the water,
remove thermometer before reaction
Explain using collision theory the effect of a catalyst on the rate of reaction
A catalyst provides an alternative route of reaction with a lower activation enthalpy so a greater proportion of the particles which collide have sufficient energy to overcome the activation enthalpy and react. The frequency of the collisions remains the same but the proportion of successful collisions which lead to reaction increases increasing the rate of reaction
Name one (gaseous) catalyst often seen in reactions
NO
Can a substance still be a catalyst if is reacts in a reaction?
Yes- sometimes catalysts will react but if they are regenerated then they are still catalysts
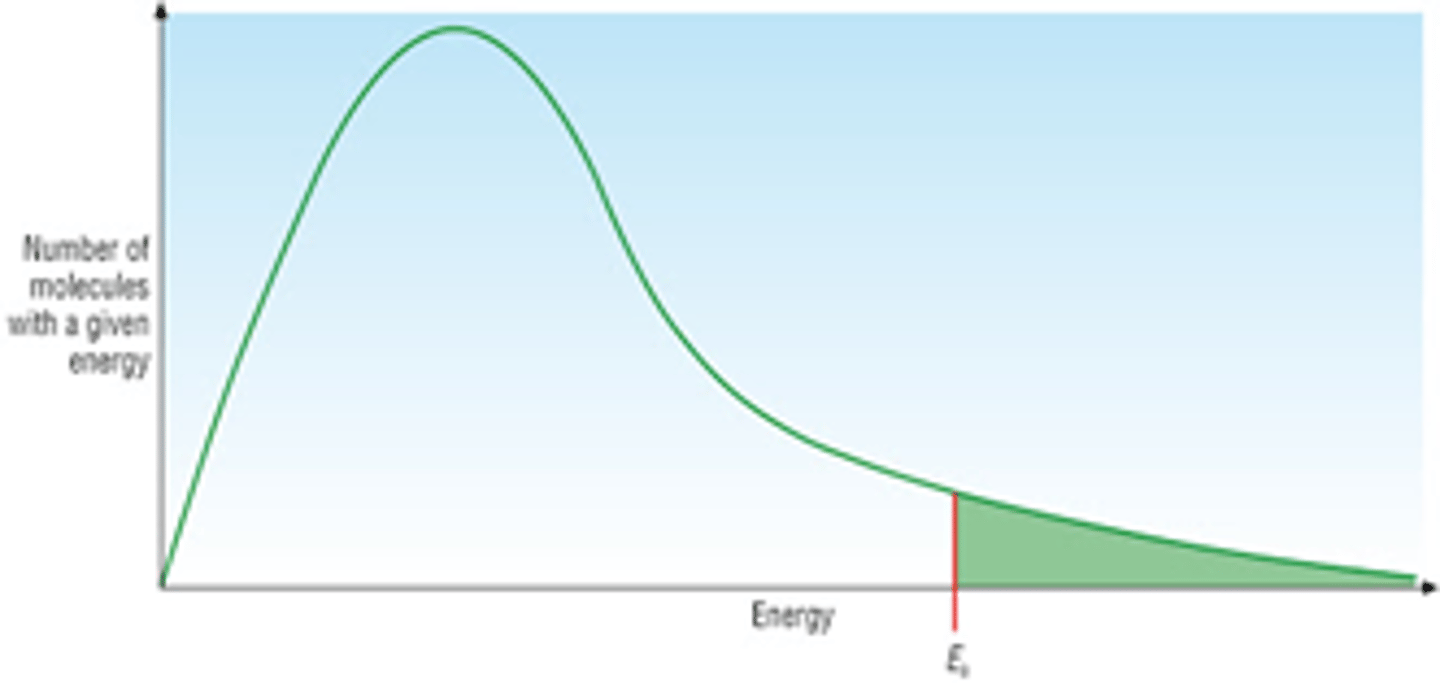
Define Boltzmann distribution
the distribution of energies of molecules at a particular temperature often shown as a graph
(it is a theoretical model developed to explain scientific observations)
What conditions does the Boltzmann distribution show the energies of molecules for?
In a gas at constant temperature
Explain the basic principles of the Boltzmann distribution
· Molecules move around inside a container colliding with each other and the walls of their container, as molecules are moving they have kinetic energy
· Molecules in a gas don’t all have the same amount of energy
· Collisions are assumed to be elastic- when they collide no energy is lost from the system
Draw a basic diagram of the Boltzmann distribution
EXAM TIP- will often see this graph in exams, may have to draw it so learn the labels and practise drawing it
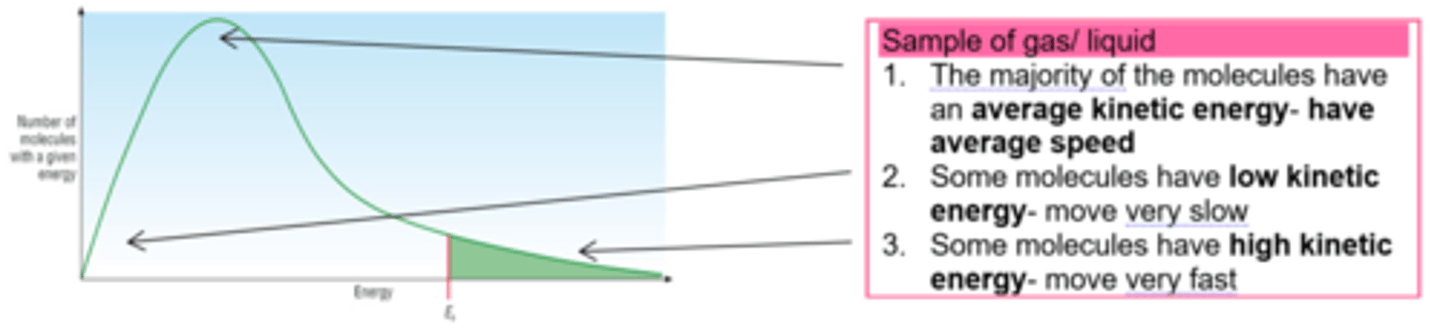
What three main energy categories can a molecule have in a sample of gas or liquid, show this on a diagram?
Sample of gas/ liquid
1. The majority of the molecules have an average kinetic energy- have average speed
2. Some molecules have low kinetic energy- move very slow
3. Some molecules have high kinetic energy- move very fast
Explain 4 important features of the Boltzmann distribution diagram
· Area under the curve- the total number of molecules in the sample, so the area does NOT change with conditions
· There are no molecules in the system with zero energy- the curve starts at the origin (0,0)
· There is no maximum energy for a molecule- curve gets close but does not touch or cross the energy axis
· Activation energy- Only the molecules with an energy greater than the activation energy Ea are able to react (and collide with correct orientation)- this is why not all collisions lead to reactions
EXAM TIP- when drawing the Boltzmann distribution which axis must the curve not touch?
Curve must NOT touch the x-axis (there is no maximum energy for the molecules)
EXAM TIP- What may exam questions often ask you to link the Boltzmann distribution to?
Enthalpy profile diagrams
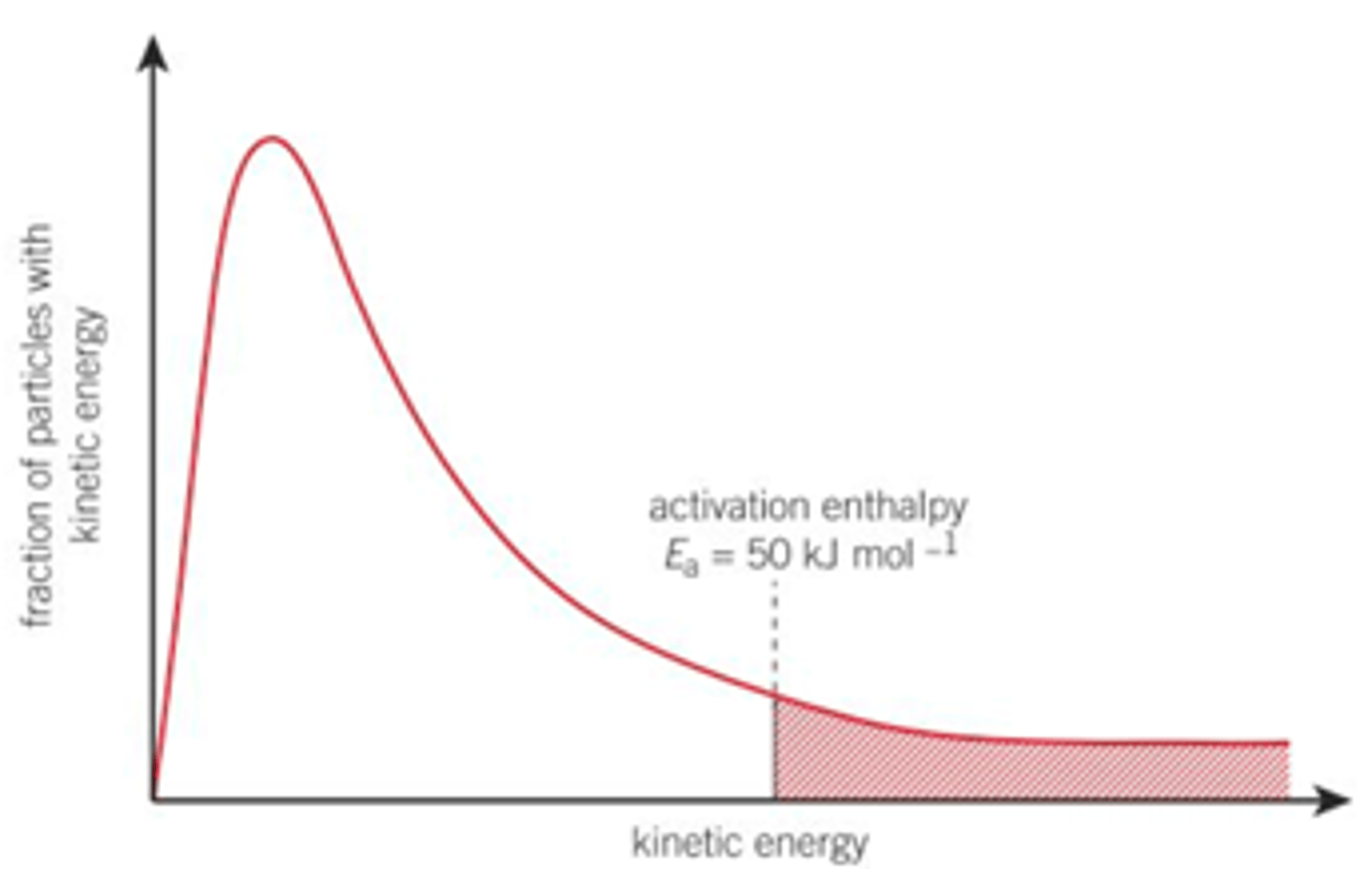
What is the activation enthalpy which is typical of many reactions?
50kJmol-1
Draw a Boltzmann distribution and show the number of collisions/ particles with energy greater than the 50kJmol-1 average, which collisions on this Boltzmann distribution would be to a reaction?
Number of collisions with energy greater than 50 kJmol-1 for the reaction at 300K is given by the shaded area underneath the curve
Only those collisions with energies in the shaded area/ with particles found in the shaded area can lead to a reaction
At a higher temperature how does the proportion of molecules with energies above 50kJmol-1 change, show this on a diagram?
At a higher temperature a higher proportion of molecules have energies above 50 kJmol-1
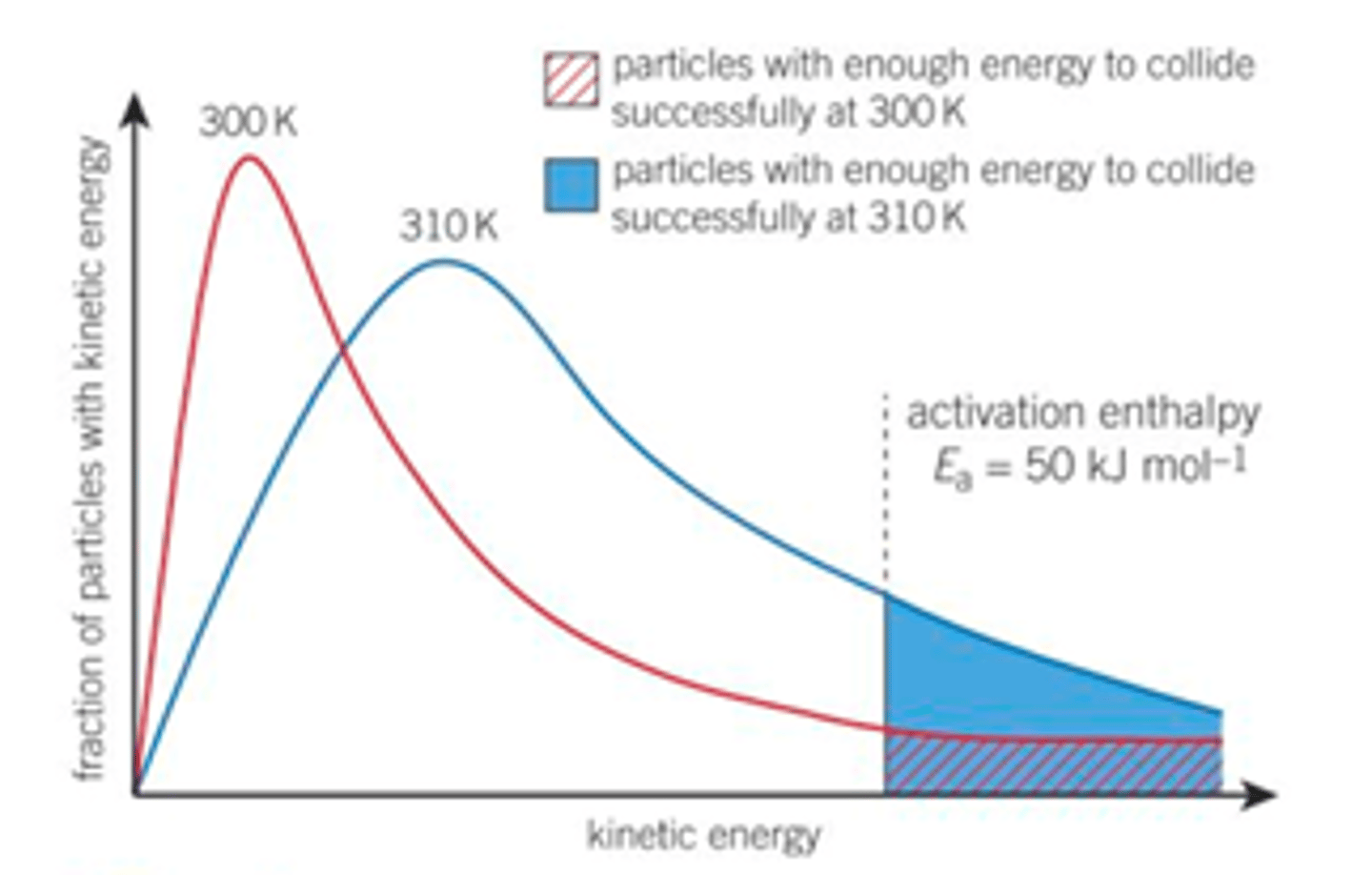
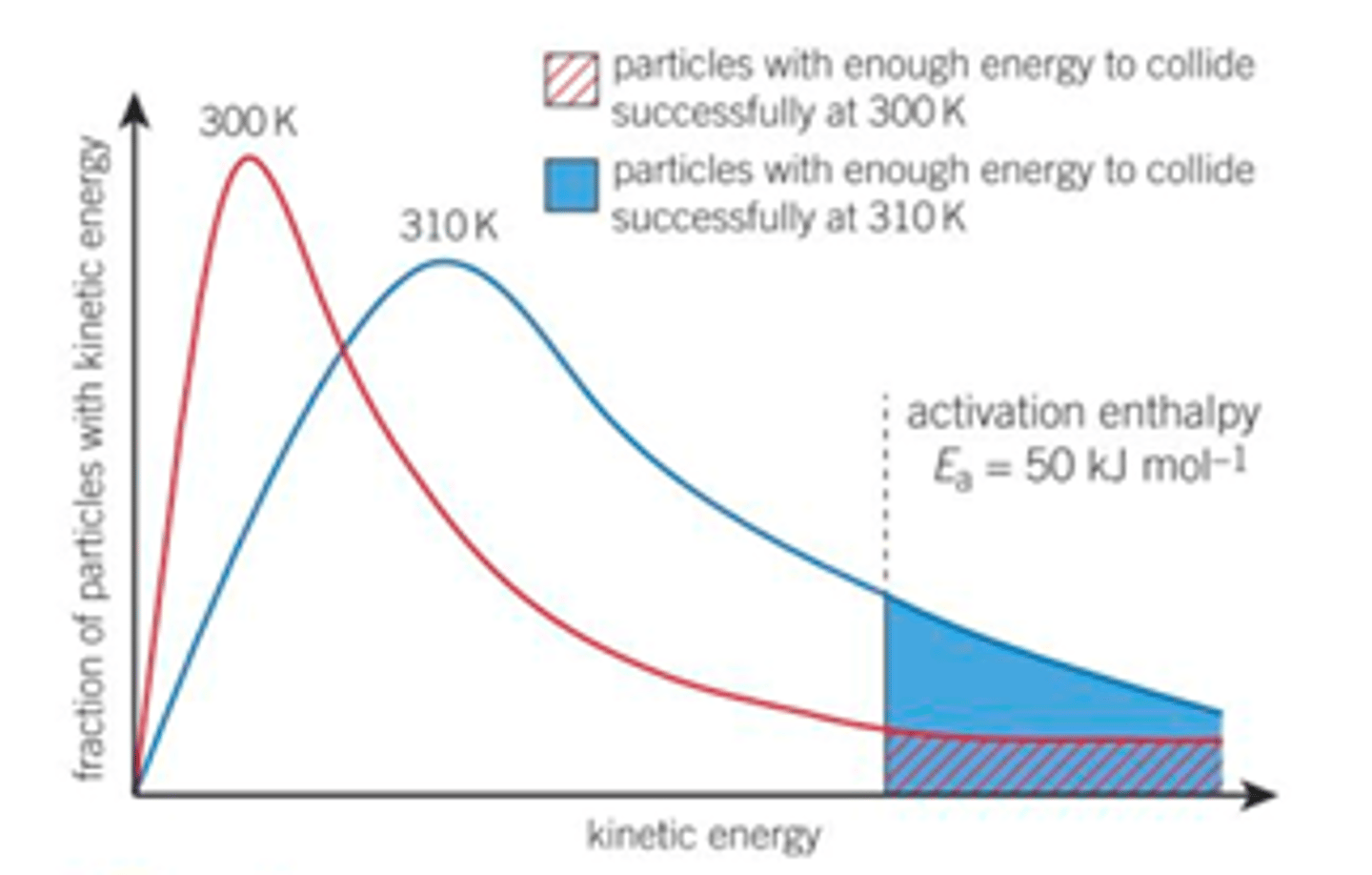
Fully explain the two reasons why a higher temperature leads to an increased rate of reaction according to the Boltzmann distribution, explain how each of these reasons changes the shape of the Boltzmann distribution curve
1. Reactions go faster at higher temperatures because a larger proportion of the colliding molecules have the minimum activation enthalpy/ energy equal to or greater than the activation energy needed to react. So on collisions a greater proportion of the molecules will overcome the activation energy of the reaction. The frequency of collisions that result in a reaction will increase- more successful collisions per unit time/ a greater proportion of/ more likely the collisions will lead to a chemical reaction- faster rate of reaction.
How this changes Boltzmann distribution- pushed to right larger area contained under Ea which means more molecules have greater then or equal to the activation energy.
2. And at higher temperatures the average kinetic energy of all the particles increases and will move faster, more kinetic energy means more frequent collisions increasing reaction rate
This also changes the shape of the Boltzmann distribution curve- pushed to the right, average energy of molecule is higher, also flatter- some molecules still have very little energy
What is the general rule when there is a 10 degree C/K temperature rise and how this affects rate, what values are needed to make this general rule work exactly?
For many reactions the rate is roughly doubled by a 10oC/K temperature rise- only a rough rule, only works exactly for reactions with an activation energy of 50 kJmol-1 and for a temperature rise from 300 to 310 K
When the activation enthalpy is higher does increasing the temperature cause more or less of an effect on the rate of reaction?
The greater the activation enthalpy the greater the effect of increasing the temperature on the rate of the reaction
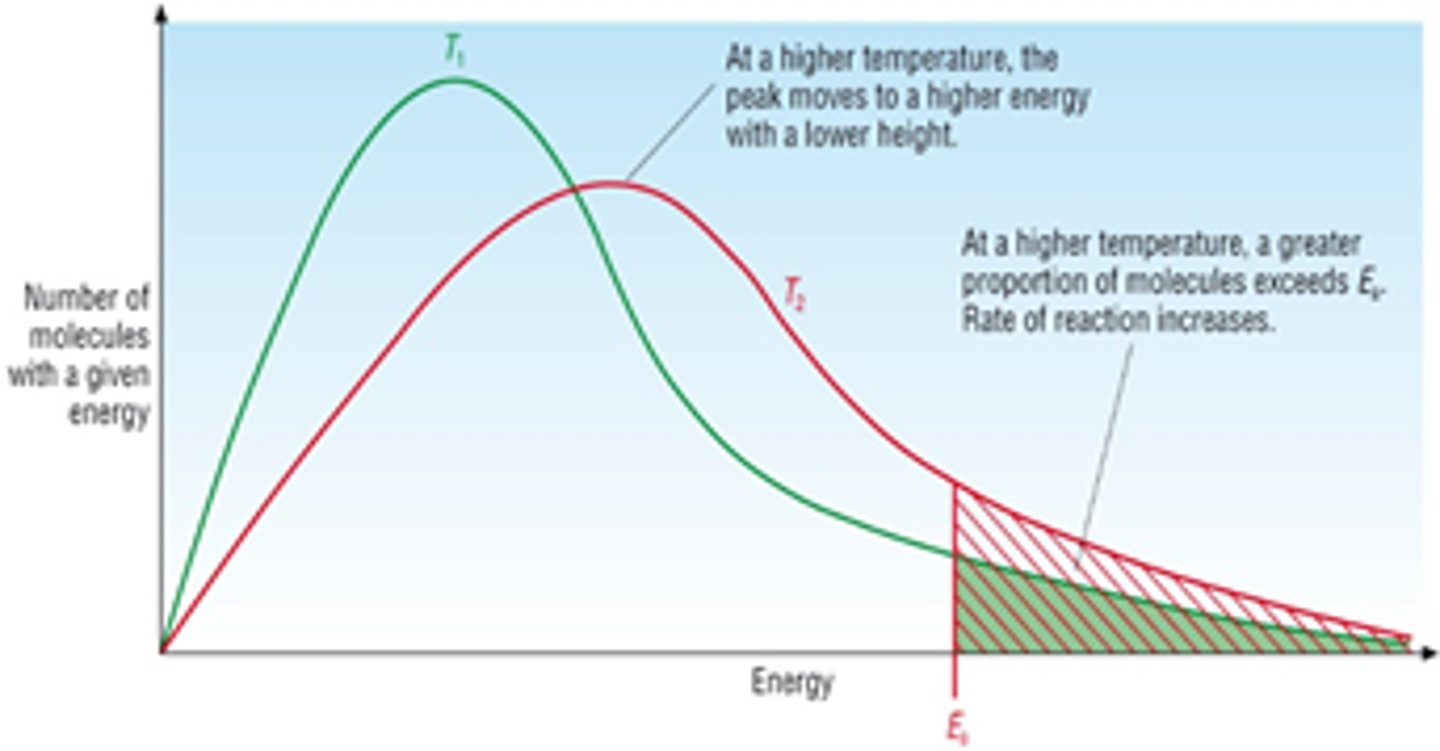
Exam Question: Describe/ Draw how the Boltzmann distribution changes at a higher temperature
· Different/ higher temperature Boltzmann distribution the peak is lower (curve flattens) and shifted to the right
· The number of molecules in the system does not change so the area under the curve remains the same- same areas so it is just squished
· (Must draw all of this to get the marks)
EXAM TIP- can be asked to sketch the graph for two different temps
Summarise how temperature affects rate in 6 points
1. Increase in temperature
2. Increases kinetic energy of molecules
3. Increases the speed of the molecules
4. Increases the collision frequency/ Increases the No of collisions with energy > activation energy
5. More successful collisions
6. Increase in the rate
EXAM TIP-instead of "more molecules" what should you say?
EXAM TIP- Instead of “more” molecules say “a greater proportion” as the number of molecules in the system stays the same
What do catalysts do?
Catalysts provide an alternative pathway for a reaction requiring less energy so with a lower activation energy to start the reaction
Describe how catalysts increase the rate of reaction
· Catalysts provide an alternative pathway for a reaction requiring less energy so with a lower activation energy to start the reaction
· At any given temperature, on collision a greater proportion molecule in the system will overcome the new lower activation energy of the reaction and area able to react
· There will be more successful collisions in a certain length of time/ a greater proportion of collisions will have sufficient energy to overcome the activation energy and be successful collisions
· The rate of reaction will increase
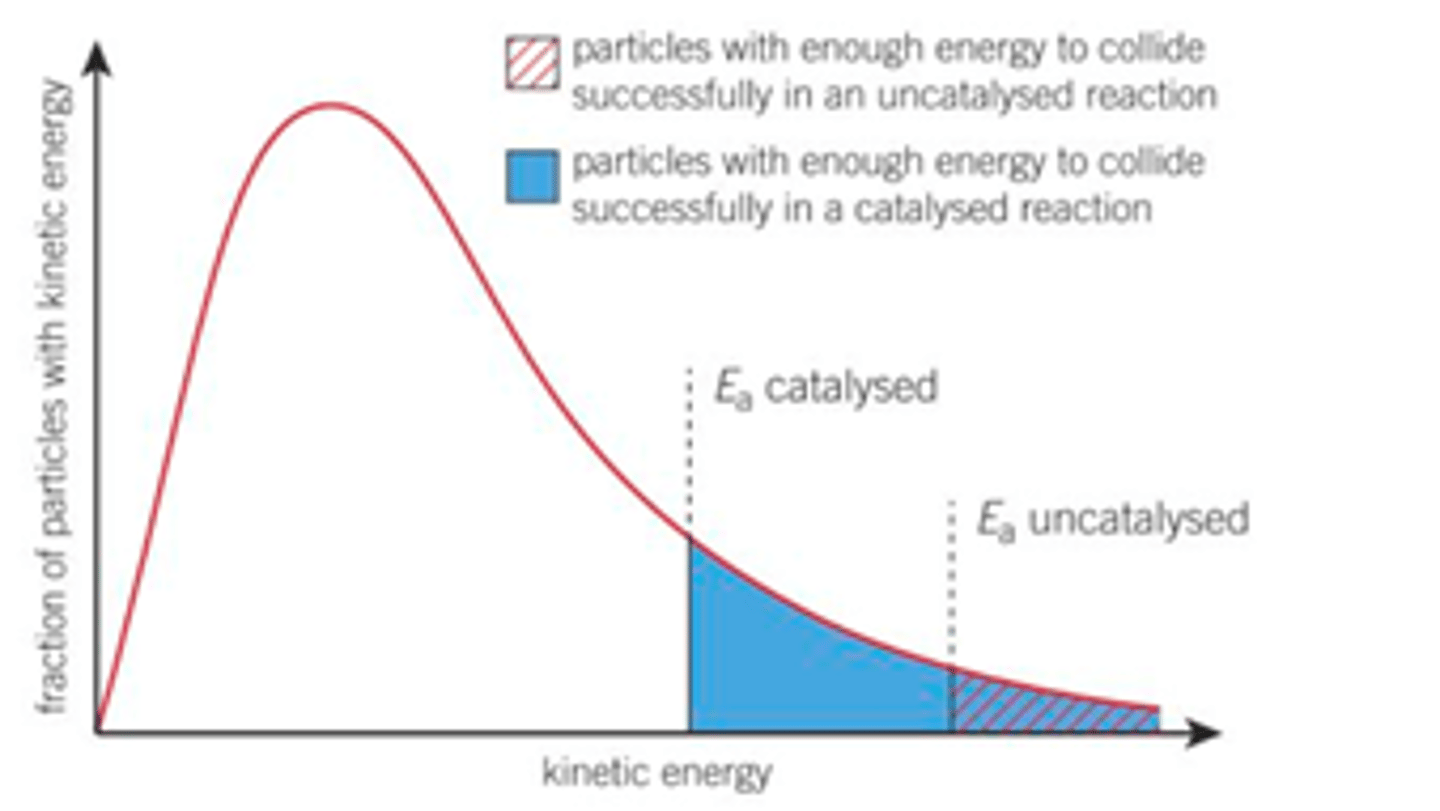
Draw the Boltzmann distribution for a catalyst
EXAM TIP- when drawing the Boltzmann distribution the energy distribution of the graph stays the same, the location of Ea changes so the area past Ea is greater- greater area means more particles have greater than or equal to activation energy
Are catalysts used up?
A catalyst increases the rate of a chemical reaction without being used up in the process
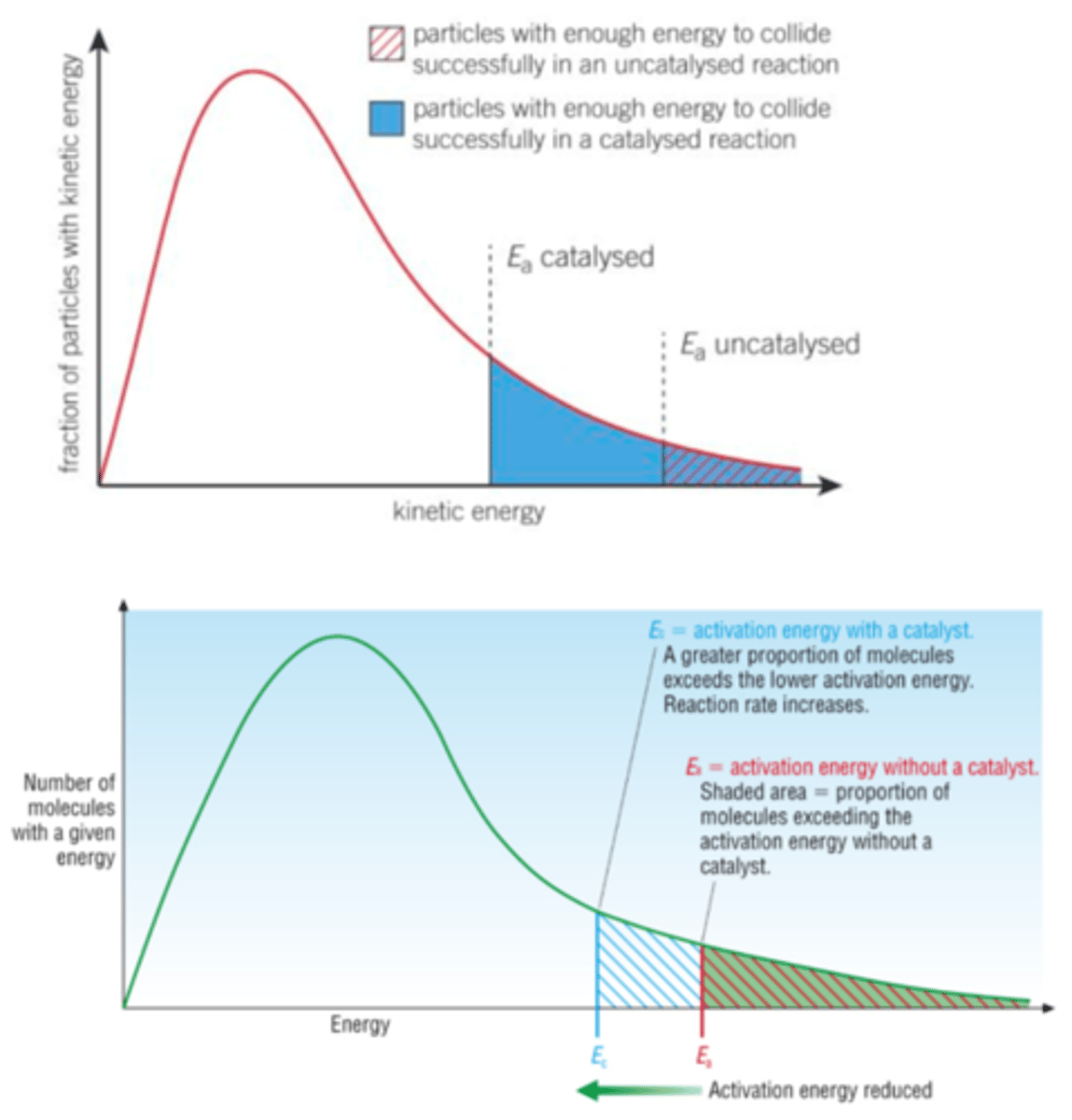
What do catalysts react form, what happens to them later in the reaction?
· A catalyst may react to form an intermediate sometimes called a transition state.
· The catalyst is later regenerated and so does not undergo a permanent change.
Do catalysts change the enthalpy of a reaction?
A catalyst does not change the enthalpy of reaction
How is a smaller activation energy shown?
A smaller activation energy is shown by a smaller maximum height/ energy of the peak
What are the advantages of using catalysts?
Catalysts affect conditions, often requiring lower temperatures, reducing energy demand and emissions
Catalysts enable different reactions to be used, with better atom economy and reduced waste