Infectious disease mod 1-3
0.0(0)
Card Sorting
1/188
There's no tags or description
Looks like no tags are added yet.
Last updated 10:02 PM on 9/8/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
189 Terms
1
New cards
Disease
harmful alteration to physiological or metabolic state of host
2
New cards
Infectious disease
Harmful alteration to physiological or metabolic state of host caused by pathogen or its product
3
New cards
Pathogen (endogenous vs exogenous)
disease causing organism/agent
* Endogenous = present in human body (cause disease when immune system becomes weak)
* Exogenous = present in external environment
* Endogenous = present in human body (cause disease when immune system becomes weak)
* Exogenous = present in external environment
4
New cards
Toxins
Soluble substances which alter normal metabolism of hosts w deleterious effects
5
New cards
Limitations of Koch’s postulates
Essentially the microorganism is found in and can be isolated from diseased organisms + when infect other organisms, causes same disease
\
Limited in that M. leprae cannot be easily culture, some viruses can only be identified by molecular techniques + a disease can be caused by more than one type of pathogen.
\
Limited in that M. leprae cannot be easily culture, some viruses can only be identified by molecular techniques + a disease can be caused by more than one type of pathogen.
6
New cards
How do infectious diseases fit into the global leading causes of death>
lower respiratory infections, neonatal conditions + diarrhoeal diseases
In low SES, infectious diseases are more spread (note that reporting of disease in low SES is dodgy)
In low SES, infectious diseases are more spread (note that reporting of disease in low SES is dodgy)
7
New cards
Deaths vs DALYs of infectious diseases?
IDs are very prevalent in DALYs (more so than causes of death where chronic diseases like IHD + COPD are major contributors)
8
New cards
Why is infectious disease impact driven by $$$?
Disease impact is affected by environment, host + pathogen.
Environment factors including health care access, intervention control programs, communication/education, shelter, sanitation/access to clean water
Environment factors including health care access, intervention control programs, communication/education, shelter, sanitation/access to clean water
9
New cards
Describe TB infection vs disease
TB infection - latent infection, asymptomatic, cannot spread, not sick, bacteria are alive but inactive + tests usually come back positive
\
TB disease- tests come back positive w an abnormal chest X ray, TB bacteria are active + usually sick -→ spread TB
\
TB disease- tests come back positive w an abnormal chest X ray, TB bacteria are active + usually sick -→ spread TB
10
New cards
Five classes of pathogens
Bacteria
Viruses
Protzoan
Helminths
Fungi
Viruses
Protzoan
Helminths
Fungi
11
New cards
Describe 5 sources of infection
Physical contact w person (i.e. STDs or skin infection)
Contact w fomites
Ingesting infected food or drink
Entry of soil or dust into wound
Bites by insects
Unsterile medical procedures
Infection carried by mother’s bloodstream
Self-infection - poor hygiene
Contact w fomites
Ingesting infected food or drink
Entry of soil or dust into wound
Bites by insects
Unsterile medical procedures
Infection carried by mother’s bloodstream
Self-infection - poor hygiene
12
New cards
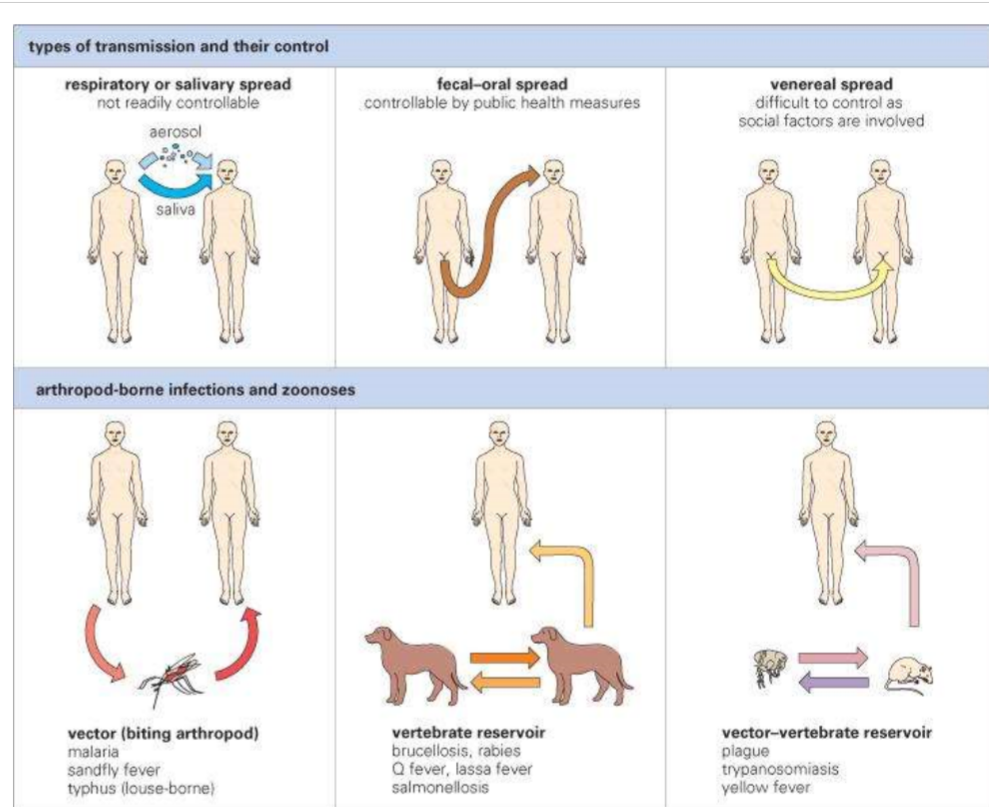
Describe entry stage of infectious disease
Transmission = look here to prevent infection
* Contacts like fomites, vectors, food, water, vertical transmission, direct physical contact
* Inhalation is most frequent source= breathing, speaking etc. (1-4m) - could lead to contaminated food or fomites
* Vertical transmission - congenital, perinatal (during birth), post natal
* Horizontal - person, vector, vertebrae to humans (rabies)
* Contacts like fomites, vectors, food, water, vertical transmission, direct physical contact
* Inhalation is most frequent source= breathing, speaking etc. (1-4m) - could lead to contaminated food or fomites
* Vertical transmission - congenital, perinatal (during birth), post natal
* Horizontal - person, vector, vertebrae to humans (rabies)
13
New cards
Purpose of pasteurised milk
Get rid of diseases like brucella, M. bovis, coxiella burnetii by heating for 72 degrees for over 15 seconds + then cooling
14
New cards
Entry
Ingress (inhalation or ingestion), penetration (through epithelial or barrier/mucous membrane- influenza virus, insect bites, cuts/wounds, organ transplant)
* Reliance on virulence factors for entry + colonisation
* i.e. pili help adhere and stop clearance
* Reliance on virulence factors for entry + colonisation
* i.e. pili help adhere and stop clearance
15
New cards
Multiplication + Spread
Understanding how to prevent infection progressing
What is needed for survival?
* Temperature (particular parts of body are infected)
* nutrients (i.e. iron)
\
Immune evasion methods?
* microbial sanctuary
* Virulence factors
What is needed for survival?
* Temperature (particular parts of body are infected)
* nutrients (i.e. iron)
\
Immune evasion methods?
* microbial sanctuary
* Virulence factors
16
New cards
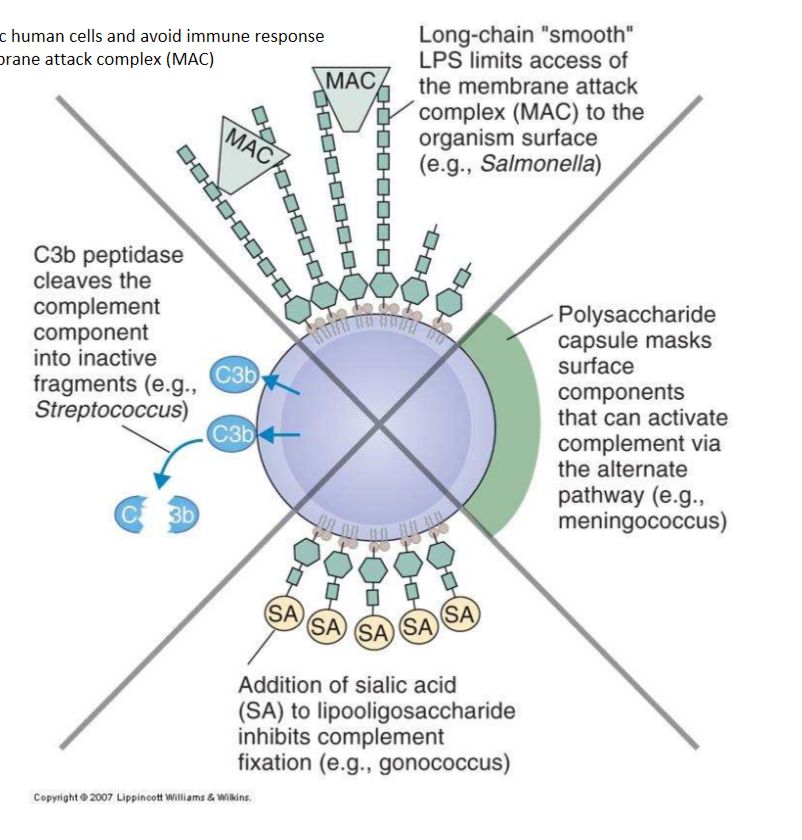
How can micro-organisms evade the complement system?
1. Prevent activation (mask- capsular polysaccharide or cleave the C3 peptidase)
2. Inhibition complement fixation (capsules- w sialic acid, or contain other sialic acid resides on LPS)
3. Inhibit access of MAC - LPS on bacteria like E. coli prevents access to surface of bacterium
17
New cards
Virus immune evasion methods (multiplication + spread)
Inhibit recognition (nucleic acid decoys + viroceptors that block cytokines)
Inhibit complement components (block C3 convertase and encode proteases)
Intracellular location + prevent NK cell recognition
Prevention of infected host cell apoptosis + antigen presentation
Inhibit complement components (block C3 convertase and encode proteases)
Intracellular location + prevent NK cell recognition
Prevention of infected host cell apoptosis + antigen presentation
18
New cards
How can microbes cause damage?
Directly- toxins + mechanical disruption/ damage
* Cholera toxin causes diarrhoea
* Clostridium botulin
* Tetanu stoxin - spastic paralysis (inhibits GABA)
* Bordetella pertussis
* IDK the mechanical disruption example….
Indirect- immune system induced (inflammation, fluid collection, swelling)
* Cholera toxin causes diarrhoea
* Clostridium botulin
* Tetanu stoxin - spastic paralysis (inhibits GABA)
* Bordetella pertussis
* IDK the mechanical disruption example….
Indirect- immune system induced (inflammation, fluid collection, swelling)
19
New cards
Types of toxins
AB toxins - intracellular target
Superantigens- excessive activation of immune system (ETEC enterotoxin)
Anthrax= 2A+B
Superantigens- excessive activation of immune system (ETEC enterotoxin)
Anthrax= 2A+B
20
New cards
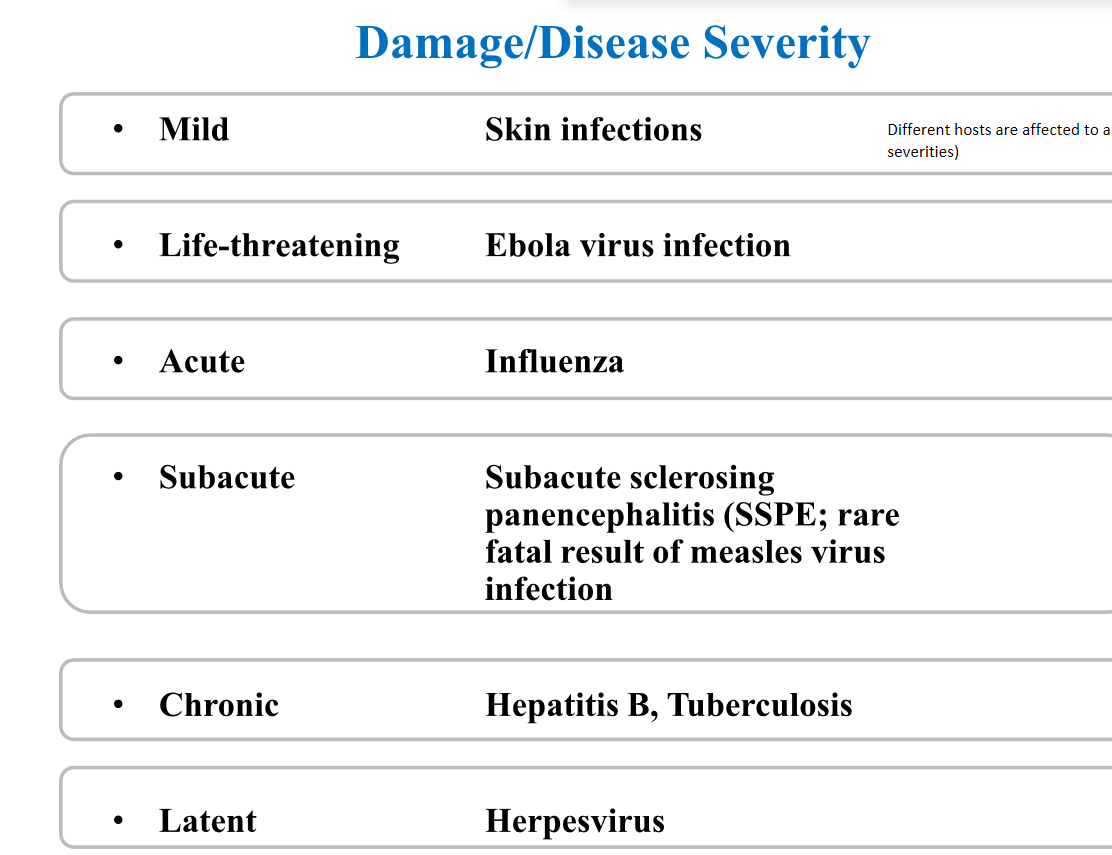
Different levels of damage/disease severity
21
New cards
Factors affecting infection outcome
Dosage (quantity) + quality of infectious organisms, including virulence (which quasispecies)
Age of host
Immunity, immunocompetence, health status
Nutritional status
Genetics
Behaviour (seeking health care)
Social determinants (availability of health care + clean water)
Age of host
Immunity, immunocompetence, health status
Nutritional status
Genetics
Behaviour (seeking health care)
Social determinants (availability of health care + clean water)
22
New cards
Functions of innate immunity
Recognise microbes encountered by host
Prevent infection/disease by eliminating microbes or allowing them to exist on body surfaces as normal, non-harmful flora
Initiate + influence nature of adaptive immune response depending on invading microbe
Activate adaptive immune response
Prevent infection/disease by eliminating microbes or allowing them to exist on body surfaces as normal, non-harmful flora
Initiate + influence nature of adaptive immune response depending on invading microbe
Activate adaptive immune response
23
New cards
Innate immunity components
Cellular - epithelial cells, phagocytic cells, pro-inflammatory cells, NK cells, APCs
Humoral - antimicrobial peptides, complement, cytokine, chemokines, acute phase proteins
Inflammation - vasodilation, vascular permeability, chemotaxis
Humoral - antimicrobial peptides, complement, cytokine, chemokines, acute phase proteins
Inflammation - vasodilation, vascular permeability, chemotaxis
24
New cards
Describe barriers of the skin, gut, lungs + eyes/nose (3x categories)
Skin- epithelial cell tight junctions + air flow (mechanical), antibacterial peptides + fatty acids in sweat (chemical), normal flora (microbiological)
\
Gut- epithelial tight junctions, flow of fluid (mechanical), low pH, enzymes (pepsin) + antibacterial peptides (chemical) + normal flora
\
Lungs- movement of cilia + epithelial cells (mechanical), antibacterial peptides (chemical)
\
Eyes/nose- mucus/hair in nose (mechanical), salivary enzymes (lysozyme) (chemical)
\
Gut- epithelial tight junctions, flow of fluid (mechanical), low pH, enzymes (pepsin) + antibacterial peptides (chemical) + normal flora
\
Lungs- movement of cilia + epithelial cells (mechanical), antibacterial peptides (chemical)
\
Eyes/nose- mucus/hair in nose (mechanical), salivary enzymes (lysozyme) (chemical)
25
New cards
Benefits of normal microbiota
Prevent attachment by pathogens
Antimicrobial factors
Biochemical reactions that benefit host (i.e. metabolise food0
Metabolic breakdown/synthesis (use of vitamin K)
Promote gut associated lymphoid tissue (GALT)
Antimicrobial factors
Biochemical reactions that benefit host (i.e. metabolise food0
Metabolic breakdown/synthesis (use of vitamin K)
Promote gut associated lymphoid tissue (GALT)
26
New cards
What are some ways that microorganisms can switch from mutualistic/commensal to a disease associated microbe/state?
Damage to epithelium (allow entry of skin flora into blood stream)
Presence of a foreign body
Transfer of microbiota to unnatural sites (self innoculation?)
Suppression of immune system by drugs or radiation
Impairment of host defenses due to infection by exogenous pathogens
Disruption of normal microbiota by antibiotics
\
Presence of a foreign body
Transfer of microbiota to unnatural sites (self innoculation?)
Suppression of immune system by drugs or radiation
Impairment of host defenses due to infection by exogenous pathogens
Disruption of normal microbiota by antibiotics
\
27
New cards
3 ways innate immune system recognises dangerous vs non dangeorus microbes?
PRR (pattern recognition receptors)
Danger signals from damaged tissue
Detection of missing self
Danger signals from damaged tissue
Detection of missing self
28
New cards
Describe common PAMPs
Highly conserved structures in microbes (not humans)
* LPS
* Peptidoglycan
* Mannose
* Flagellin
* Pilin
PRR bind, allow immediate response (responses 1000 molecule patterns)
* LPS
* Peptidoglycan
* Mannose
* Flagellin
* Pilin
PRR bind, allow immediate response (responses 1000 molecule patterns)
29
New cards
What is a subset of PRRs?
Toll like receptors-
TLR has two subsets-
* subset one is present on membrane surface (other cell membrane PRRs include Cd14, mannose receptor etc.)
* subset two is present within cell (endosome prrs)- detect DNA and RNA
\
Cytoplasmic PRRs
* cell cytoplasm - think NOD-like receptors
Soluble/secreted PRRs
* enable or enhance response to host cells microbial products
TLR has two subsets-
* subset one is present on membrane surface (other cell membrane PRRs include Cd14, mannose receptor etc.)
* subset two is present within cell (endosome prrs)- detect DNA and RNA
\
Cytoplasmic PRRs
* cell cytoplasm - think NOD-like receptors
Soluble/secreted PRRs
* enable or enhance response to host cells microbial products
30
New cards
Induced innate immunity response to infection
increase production of antimicrobial peptides
secretion of mediators of inflammation
activation of complement
clotting cascade
chemotactic attraction of phagocytic cells
inflammation
secretion of mediators of inflammation
activation of complement
clotting cascade
chemotactic attraction of phagocytic cells
inflammation
31
New cards
What is a broad spectrum antibiotic
Covers most bacteria- use when not sure what pathogen or injury to mechanical barrier
32
New cards
Functions of the complement system
Opsonisation
Inflammation
Chemotaxis
Phagocytic cells activated
Lysis of some microbes directly
Inflammation
Chemotaxis
Phagocytic cells activated
Lysis of some microbes directly
33
New cards
Anaphylatoxin
Substance produced during complement activation that is a mediator of inflammation by binding to mast cells, basophils + platelets
34
New cards
What is the MAC?
Membrane attack complex (MAC) -→ lyses gram negative bacteria, envelope viruses + foreign cells
Phagocytosis resistant bacteria (such as meningococci + gonococci)
Phagocytosis resistant bacteria (such as meningococci + gonococci)
35
New cards
Regulate complement activation
Complement system can easily destroy cells so much be regulated
* Can self-amplify but each component can be inhibited
* Suicide substrate mechanism- covalent bond formed w active site
* Proteolytic digestion of active fragments
\
* Can self-amplify but each component can be inhibited
* Suicide substrate mechanism- covalent bond formed w active site
* Proteolytic digestion of active fragments
\
36
New cards
Chemokines vs cytokiens
Cytokines = secreted from pro-inflammatory cells (macrophages + lymphocytes)
\
Chemokines = type of cytokine that has chemotactic properties (produced by macrophages, endothelial + epithelial cells)
\
Chemokines = type of cytokine that has chemotactic properties (produced by macrophages, endothelial + epithelial cells)
37
New cards
Chemotaxis
Cells moving in response to chemotaxins
* Exogenous (produced by bacteria)
* Endogenous (complement, chemokines, leukotrienes)
* phagocytes are directly influenced by chemotaxins
* Exogenous (produced by bacteria)
* Endogenous (complement, chemokines, leukotrienes)
* phagocytes are directly influenced by chemotaxins
38
New cards
Describe neutrophils
Bone marrow production
Motile + **phagocytic**
A PMN
Have azurophils w anti-microbial defensins that fuse w phagocytic vacuoles
Circulates in blood
Short lived + numerous, first at site of microbe invasion
Do not use mitochondria for energy= glycogen stores
Form pus when die
Motile + **phagocytic**
A PMN
Have azurophils w anti-microbial defensins that fuse w phagocytic vacuoles
Circulates in blood
Short lived + numerous, first at site of microbe invasion
Do not use mitochondria for energy= glycogen stores
Form pus when die
39
New cards
What are the PMNs?
Polymorphonuclear leukocytes = segmented nucleus
\
They are BEN - basophils, eosinophils + neutrophils (also granulocytes)
\
They are BEN - basophils, eosinophils + neutrophils (also granulocytes)
40
New cards
Describe eosinophils
Not good phagocytes but exocytose granules
Target animal parasites (protozoa + worms- these organisms are too large to be phagocytosed)
Target animal parasites (protozoa + worms- these organisms are too large to be phagocytosed)
41
New cards
Monocytes + macrophage immune response
Slower than PMNs, settle in tissue (resident tissue macrophages)
* share common progenitor w neutrophils
* Differentiate after leaving bone marrow
* Important in innate + adaptive immunity
* Neutrophils recruit
* share common progenitor w neutrophils
* Differentiate after leaving bone marrow
* Important in innate + adaptive immunity
* Neutrophils recruit
42
New cards
How are macrophages activated?
LPS on PRRs
Platelet activating factor
Cytokines (think IFN-gamma)
Fibronectin (acute phase protein)
Platelet activating factor
Cytokines (think IFN-gamma)
Fibronectin (acute phase protein)
43
New cards
Function of cytokines released by macrophages
IL-1beta - activates vascular endothelium inducing fever + IL-6
TNF-alpha - increase vascular permeability
IL-6- increased antibody production
CXCL-8- chemotaxin (basophils, neutrophils)
IL-12 - NK differentiation
TNF-alpha - increase vascular permeability
IL-6- increased antibody production
CXCL-8- chemotaxin (basophils, neutrophils)
IL-12 - NK differentiation
44
New cards
What events does the macrophage induce?
Cytokines released induce vascular permeability -→ leukocytes move into vessels from increased adherence factors → leukocytes extravasate (move out of circulatory system) -→ blood clotting in micro-vessels
\
Diapedesis - outward passage of WBC through intact vessel walls to surrounding tissues
\
Diapedesis - outward passage of WBC through intact vessel walls to surrounding tissues
45
New cards
How do monocytes and macrophages kill?
Clear remains of microorganisms and neutrophils -→ phagocytose debris
Differentiate, complement components and cytokines
Activated macrophages- phagocytose better, take up more oxygen + secrete more hydrolytic enzymes
\
Activated by C3b + interferon gamma
Differentiate, complement components and cytokines
Activated macrophages- phagocytose better, take up more oxygen + secrete more hydrolytic enzymes
\
Activated by C3b + interferon gamma
46
New cards
Describe mastocytes
Mast cells - in locations that are in close contact w external environment (skin, airways, intestines)
Granules- histamine, heparin + serotonin
* Release of granules after C3a and C5a trigger = vasodilation and chemotaxis
* Can also phagocytose + use ROS
\
3 mediators
* Granule releases -→ vasodilation, capillary permeability, chemokinesis + bronchoconstriction
* Lipoxygenase pathway(leukotrienes)- bronchoconstriction, chemotaxis + chemokinesis
* Cyclooxygenase pathway - prostaglandins + thromboxane = platelet aggregation + alter vasodilation
Granules- histamine, heparin + serotonin
* Release of granules after C3a and C5a trigger = vasodilation and chemotaxis
* Can also phagocytose + use ROS
\
3 mediators
* Granule releases -→ vasodilation, capillary permeability, chemokinesis + bronchoconstriction
* Lipoxygenase pathway(leukotrienes)- bronchoconstriction, chemotaxis + chemokinesis
* Cyclooxygenase pathway - prostaglandins + thromboxane = platelet aggregation + alter vasodilation
47
New cards
Outline the steps of phagocytosis
1. Recognition + attachment (i.e. PAMPs, chemotaxins, opsonins)
2. Engulfment (uptake into a phagosome via actin filaments)
3. Killing + degradation (lysosome fuses, lysosomal enzymes- ROIs and NO)
1. Oxygen dependent killing (hydrogen peroxide)
2. Oxygen independent killing (toxic products)
48
New cards
Oxygen dependent killing
In phagolysosome - NADPH oxidase (produce hydrogen peroxide= bactericidal, particularly for gram positive in skin and upper respiratory)
49
New cards
Oxygen independent killing
Microbe killing in anaerobic conditions - using toxic products or enzymes (lysozymes, phospholipase A, neutral proteases, cathelicidin)
50
New cards
Histological process of inflammation
1. Dilation of arterioles, capillaries + venules (inflammatory mediators)
2. Increased permeability + blood flow (more antimicrobial cell migration)
3. Exudation of fluids (plasma proteins)
4. Leukocyte migration into inflammatory focus
51
New cards
Characteristics of inflammation
Heat
Redness
Swelling
Pain
Loss of function
Redness
Swelling
Pain
Loss of function
52
New cards
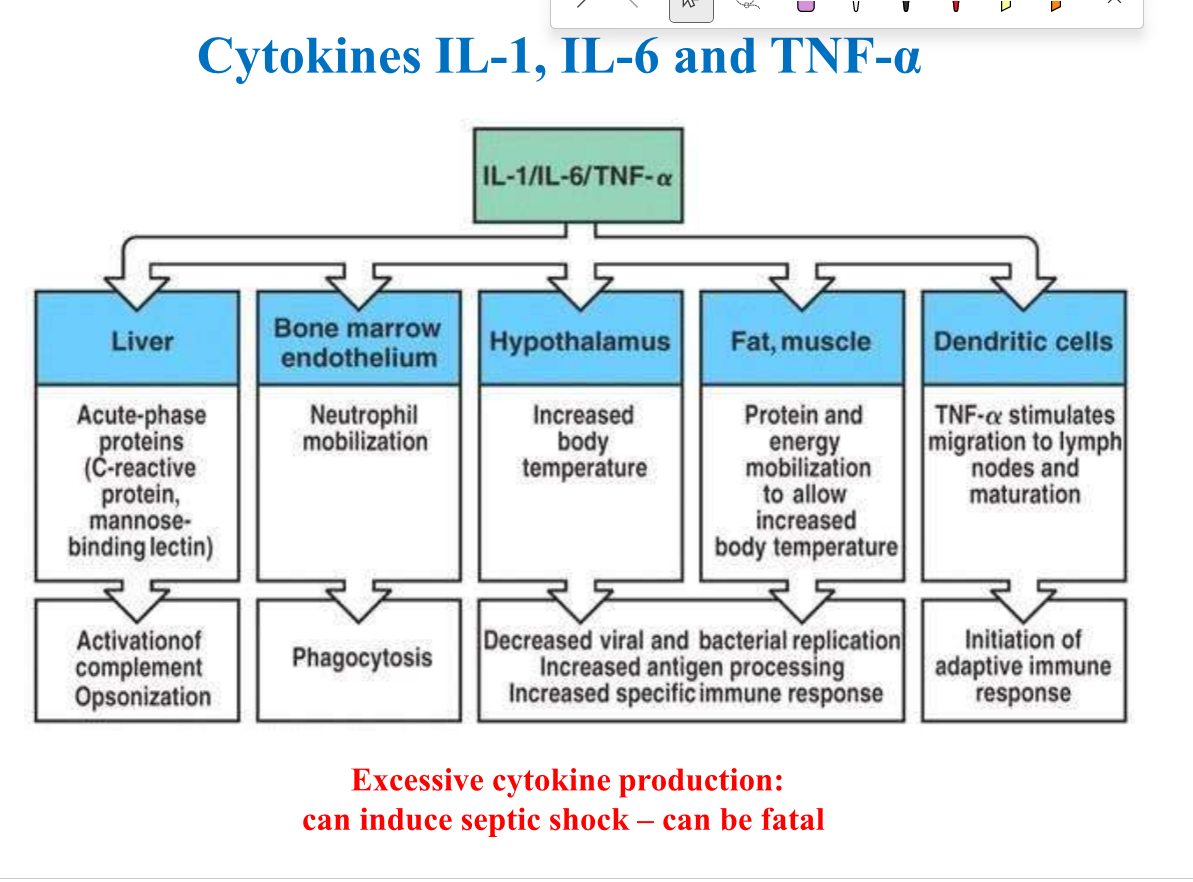
Function of IL-1, IL-6 and TNF-alpha
Induce acute phase response (opsonisation+ complement activation)
Neutrophil mobilisation
Increased temperature
Increase protein/energy mobilisation
Dendritic cells
Neutrophil mobilisation
Increased temperature
Increase protein/energy mobilisation
Dendritic cells
53
New cards
Acute phase response
CRP, serum amyloid P, fibrinogen = induced by pro-inflammatory cytokines, beings after inflammation (protects host)-→ helps active complement
54
New cards
Sepsis
Systemic inflammatory response syndrome in reaction to infection (usually bacteria) - 30% mortality
C reactive protein
C reactive protein
55
New cards
Fever
Elevation of temp due to inflammatory mediators (pyrogens)
* Some leukocytes function better at higher temperatures
* Endogens pyrogens decrease metals required for some bacterias’ growth
* Directly can inhibit some microbes (pneumococci + gonococci)
Cons
* metabolic demands, CV stress (CHF or IHD)
* Too many pyrogens leads to sepsis, tissue necrosis, organ failure, shock + death
* Some leukocytes function better at higher temperatures
* Endogens pyrogens decrease metals required for some bacterias’ growth
* Directly can inhibit some microbes (pneumococci + gonococci)
Cons
* metabolic demands, CV stress (CHF or IHD)
* Too many pyrogens leads to sepsis, tissue necrosis, organ failure, shock + death
56
New cards
What are the three ways a body can respond to a virus
1. Destroying virus
2. Destroying virus infected host cell
3. Protecting un infected cells from becoming infected
57
New cards
NK cells + how tey function
Cytotoxic activity- not specific, must be activated by adaptive responses, kill virus infected cells w lytic granules
* Detect changed self cells
* Use Natural kill receptors - bind to glycoproteins on cells
* Killer inhibitory receptors- stop killing normal host cells of can bind to MHC class I molecules
Granule released onto non-self cell
* perforin (insert itself into membrane of target cell)
* Granzymes (serine proteases that cleave and active intracellular caspases)
* Detect changed self cells
* Use Natural kill receptors - bind to glycoproteins on cells
* Killer inhibitory receptors- stop killing normal host cells of can bind to MHC class I molecules
Granule released onto non-self cell
* perforin (insert itself into membrane of target cell)
* Granzymes (serine proteases that cleave and active intracellular caspases)
58
New cards
APCs cell types
dendritic cells + macrophages
59
New cards
Why is the adaptive immune system required?
Most pathogens have developed defenses to partially escape destruction by innate protective mechanism- need specific + strong mechanisms
60
New cards
Summary of immune innate system
61
New cards
What are potential outcomes of the immune response?
Successful removal of all pathogens + development of immunity
Contained infection but not eliminated (TB infection, HIV, herpes)
Pathogen or immune response or both leave tissue damage (disease)
Contained infection but not eliminated (TB infection, HIV, herpes)
Pathogen or immune response or both leave tissue damage (disease)
62
New cards
Vectors
Living transmitters of pathogens- usually arthropods
* anopheles- transmits malaria parasites
* Tsetse flies- sleeping sickness
* Reduviid- Chagas disease
* anopheles- transmits malaria parasites
* Tsetse flies- sleeping sickness
* Reduviid- Chagas disease
63
New cards
Primitive/definitive vs secondary/intermediate vs dead end (incidental) host
Primitive= host in which the parasite reaches maturity and reproduces sexually
Secondary = host that harbours parasite during a developmental stage
Dead end = intermediate host that does not allow transmission to primary host, cannot complete development
Secondary = host that harbours parasite during a developmental stage
Dead end = intermediate host that does not allow transmission to primary host, cannot complete development
64
New cards
Toxoplasmosis
Primary/definitive host = domestic cats
Intermediate hosts = birds + rodents
Humans = dead end hosts, become infected by eating consuming cyst contaminated substances/blood
T. gondii - 95% of ppl are infected, very few have the disease
Intermediate hosts = birds + rodents
Humans = dead end hosts, become infected by eating consuming cyst contaminated substances/blood
T. gondii - 95% of ppl are infected, very few have the disease
65
New cards
Infection vs disease of parasitic infections
Parasitic infections - parasites exploit hosts to survive but does not aim to kill (cannot complete lifecycle) -→ disease then will usually be prolonged or repeated + highly burdensome
66
New cards
Anthroponosis
reverse zoonosis (human to animal),
60% of pathogens are zoonotic
60% of pathogens are zoonotic
67
New cards
What are some parasitic mechanisms for evading host immune response?
Antigenic variation - Plasmodium species
Intracellular - plasmodium
Camouflage- schistosomes
Cleavage/destroying of Igs- Giardia
Suppression or redirection of immune response- most protozoa
Surviving phagocytosis- T. cruzi
Intracellular - plasmodium
Camouflage- schistosomes
Cleavage/destroying of Igs- Giardia
Suppression or redirection of immune response- most protozoa
Surviving phagocytosis- T. cruzi
68
New cards
protozoa + potential methods of reproduction
unicellular eukaryotes- occur wherever there is water (intracellular or extracellular infections)
\
Asexual (mitotic divisions- binary/multiple fission) + sexual reproduction (gametocytes, gametes, fertilisation)
\
Asexual (mitotic divisions- binary/multiple fission) + sexual reproduction (gametocytes, gametes, fertilisation)
69
New cards
4 types of protozoa
Amoebae
Flaggelates
Ciliates
Sporozoa
Flaggelates
Ciliates
Sporozoa
70
New cards
Intracellular vs extracellular protozoan infectiosn
Intracellular (plasmodium or leishmania)- usually spread through body via RBCs or macrophages, need a vector for transmission (can’t survive well outside of host)
Extracellular (giarda) - have an active trophozoite form + a dormant cyst form (resistant to drying + acidic pH- good for transmission)
Extracellular (giarda) - have an active trophozoite form + a dormant cyst form (resistant to drying + acidic pH- good for transmission)
71
New cards
Different protozoan lifecycles
Direct
Faecal-oral
Vector-borne
Predator-prey
Faecal-oral
Vector-borne
Predator-prey
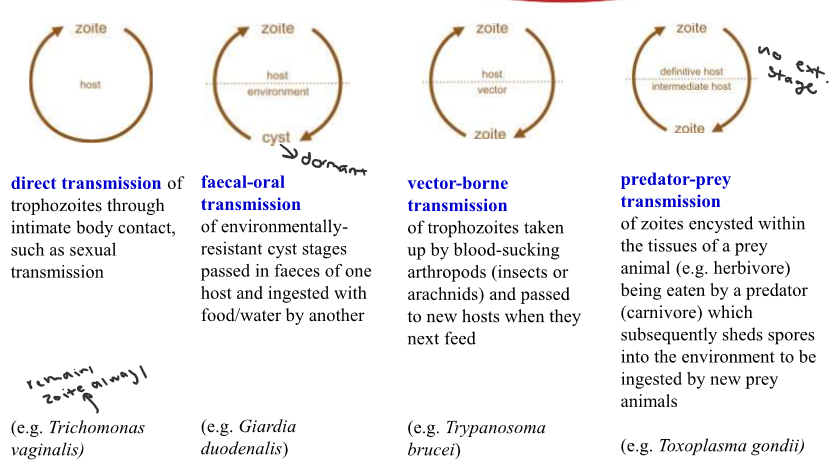
72
New cards
What cells do plasmodium infect?
Hepatocytes + erythrocytes
73
New cards
Why is malaria light microscopy advantageous?
Traditional method and can understand stage of divison (no sexual reproduction in the body) - can sometimes differentiate between species
\
P. falciparum trophozoites and schizonts are not usually seen because they attach themselves to venular endothelium
\
P. falciparum trophozoites and schizonts are not usually seen because they attach themselves to venular endothelium
74
New cards
PCRs vs RDTs for malaria diagnosis
RDTs- variation in sensitivity, high false negative rate (gene deletions of plasmodium), need cold storage _+ cannot identify all species
PCR- not used in clinical setting, expensive, more sensitive
PCR- not used in clinical setting, expensive, more sensitive
75
New cards
Discuss Malaria transmission factors
Geography and topography of land
Mosquito vector species, abundance, drug sensitivity
Temp + rainfall
Strength of immune system
Quality of housing
How ppl spend time + times when vectors are feeding
Amount + type of agriculture in area
Mosquito vector species, abundance, drug sensitivity
Temp + rainfall
Strength of immune system
Quality of housing
How ppl spend time + times when vectors are feeding
Amount + type of agriculture in area
76
New cards
What is the erythrocytic cycle + RBC preference of plasmodium - F., V., O.,+ M.
Erythrocytic cycle- A stage in the life cycle of the malaria parasite found in the red blood cells.
F- 36-48hrs, prefers younger RBCs but can infect all RBCs (high drug resistance, severe in non-immune)
Vivax- 48hrs, reticulocytes (immature RBCs, mild to severe)
Ovale- 48 hrs, reticulocytes (mild, no drug resistance)
Malariae- 72 hrs + older cells (mild, no drug resistance)
F- 36-48hrs, prefers younger RBCs but can infect all RBCs (high drug resistance, severe in non-immune)
Vivax- 48hrs, reticulocytes (immature RBCs, mild to severe)
Ovale- 48 hrs, reticulocytes (mild, no drug resistance)
Malariae- 72 hrs + older cells (mild, no drug resistance)
77
New cards
Asexual blood stage lifecycle of plasmodium
Ring form -→ trophozoites -→ schizont (last two can sequester in various organs)
\
\
78
New cards
Malaria zoonosis
P. knowelsi (long tailed macaque can be a natural host- monkeys are asymptomatic)
* Immature trophozoites can look similar to P. falciparum
* Older trophozoites + schizonts look like P. malariae
* Immature trophozoites can look similar to P. falciparum
* Older trophozoites + schizonts look like P. malariae
79
New cards
Describe severe malaria + vulnerable groups
Usually by P. falciparum in children, travellers, co-infected + pregnant women
* 80% of deaths occur in young African children
* Malarial anaemia, blood transfusions + deaths
* 80% of deaths occur in young African children
* Malarial anaemia, blood transfusions + deaths
80
New cards
Why does P. falciparum cause the most mortality?
Infects RBCs of any age
*Pf*EMP1-→binds + attaches to RBCs/walls of blood vessels (rosetting, cytoadhesion + autoagglutination)
*Pf*EMP1-→binds + attaches to RBCs/walls of blood vessels (rosetting, cytoadhesion + autoagglutination)
81
New cards
PAM
Pregnancy associated malaria-
* Can cause maternal anemia
* Fetal loss (LBW, premature, death)
* Congenital malaria (maybe, maybe not)
* Can cause maternal anemia
* Fetal loss (LBW, premature, death)
* Congenital malaria (maybe, maybe not)
82
New cards
Malaria protection + immunity
* exposure
* exposure
Repeat infections = partially protective immunity
Semi-immune = mild symptoms/asymptomatic even though can still be infected by malaria
Maternal antibodies protect newborns in first few months of life
in lower transmission areas- less infections, most ppl have no protective immunity
Semi-immune = mild symptoms/asymptomatic even though can still be infected by malaria
Maternal antibodies protect newborns in first few months of life
in lower transmission areas- less infections, most ppl have no protective immunity
83
New cards
Malaria protection + immunity
* genetic
* genetic
Protection against P. falciparum malaria
* Sickle cell trait (heterozygotes for abnormal HbS, cannot support parasite growth - 80% in SSA)
* Thalassemias (abnormal Hb formation)
* G6PD deficiency
protection against P. vivax
* Duffy blood group = vivax needs Duffy positive RBC for invasion, majority of Africans are Duffy negative (rare in SSA)
* Sickle cell trait (heterozygotes for abnormal HbS, cannot support parasite growth - 80% in SSA)
* Thalassemias (abnormal Hb formation)
* G6PD deficiency
protection against P. vivax
* Duffy blood group = vivax needs Duffy positive RBC for invasion, majority of Africans are Duffy negative (rare in SSA)
84
New cards
Malaria prevention strategies
Vaccines that exist (mosquirix, matrix) have poor efficacy -→ only used for children in affected areas
Insecticides (indoor residual spray, insecticide treated nets, increased drug resistance) + drugs (travellers, pregnant women take sulfaxoin + pyrimethamine (iPTP))
Insecticides (indoor residual spray, insecticide treated nets, increased drug resistance) + drugs (travellers, pregnant women take sulfaxoin + pyrimethamine (iPTP))
85
New cards
Issues w antimalarial drugs
Increased resistance- must use a combo
**Artemisin combination therapies** - artemisinin (short half life) { piperaquine and artemether + lumefantrine
* Cases of resistance in western cambodia
* reasons could be due to improper ACT use, substandard drugs, incomplete dosages for prophylaxis, malaria parasites w unique ability to develop any anti-malarial drug
**Artemisin combination therapies** - artemisinin (short half life) { piperaquine and artemether + lumefantrine
* Cases of resistance in western cambodia
* reasons could be due to improper ACT use, substandard drugs, incomplete dosages for prophylaxis, malaria parasites w unique ability to develop any anti-malarial drug
86
New cards
Why is chloroquine no longer used?
Used to be a drug that was rolled out for eradication and then resistance built up
Was part of an eradicaiton program (1955-1969) but they ignored signs of resistance + relied only on one strategy
Was part of an eradicaiton program (1955-1969) but they ignored signs of resistance + relied only on one strategy
87
New cards
Eradication vs elimination
Eradication = permanent reduction to zero of the worldwide incidence of infection caused by human malaria parasites as a result of deliberate efforts
\
Elimination = reduction to zero of incidence of infection caused by a parasite in a defined geographical area as a result of deliberate (need to continue measures to prevent re-establishment of transmission)
\
Elimination = reduction to zero of incidence of infection caused by a parasite in a defined geographical area as a result of deliberate (need to continue measures to prevent re-establishment of transmission)
88
New cards
How to re-establish a downward trajectory in malaria incidence and deaths?
Political commitment
Substantial funding
Regional collaboration
Coordinated global response
new tools = drugs, vaccines, vector control, education + implementation strategies
Substantial funding
Regional collaboration
Coordinated global response
new tools = drugs, vaccines, vector control, education + implementation strategies
89
New cards
Trypanosoma diseases
Trypansoma brucei/T. gambiense/T. rhodiense = African sleeping sickness (HAT)
Trypansoma cruzi = American Trypansomiasis = Chagas disease
Trypansoma cruzi = American Trypansomiasis = Chagas disease
90
New cards
Characteristics of HAT
Human african trypanosomias = can be caused by T. brucei gambiense (95%, chronic) or T. brucei rhodesiense (acute, severe)
* Hosts = most mammals (zoonosis- reservoirs of animals particularly in rhodesiense)
* nagana cattle disease pathogens cannot infect humans (not zoonotic)
* Bloodstream + CSF (**extracellular)**
* Transmitted by the bite of a tsetse fly (M&F)
* vertical transmission
* also sexual contact
* Mostly in Tropical Africa (DRC)
* Epidemiology- 2-4000 deaths per year + high DALY burden
* Hosts = most mammals (zoonosis- reservoirs of animals particularly in rhodesiense)
* nagana cattle disease pathogens cannot infect humans (not zoonotic)
* Bloodstream + CSF (**extracellular)**
* Transmitted by the bite of a tsetse fly (M&F)
* vertical transmission
* also sexual contact
* Mostly in Tropical Africa (DRC)
* Epidemiology- 2-4000 deaths per year + high DALY burden
91
New cards
Nagana Cattle disease
Also caused by T. brucei, T. congolense. T. vivax
46 million cattle are threatened, costs lots of money
46 million cattle are threatened, costs lots of money
92
New cards
Life cycle of HAT
Bite of Tsetse flie inoculates metacyclic trypomastigotes under skin→ parasite replicates in blood, lymph, spinal fluid (binary fission) as a blood stream trypomastigote → trypomastigotes in blood are taken up by tsetse flies
\
*Cannot be transmitted without fly*
Within fly- parasites become procyclic trypomastigotes in midgut, divide by binary fission, become epimastigotes in salivary gland and then form metacyclic metacyclic trypomastigotes
\
*Cannot be transmitted without fly*
Within fly- parasites become procyclic trypomastigotes in midgut, divide by binary fission, become epimastigotes in salivary gland and then form metacyclic metacyclic trypomastigotes
93
New cards
T. b. gambiense vs T. b. rhodesiense distribution + treatment
Gambiense- most cases, found in west and central Africa, chronic cases- **takes longer to develop into the menigoencephatic stage**
* fexinidazole, pentamidine
* eflornithine, NECT, fexinidazole
Rhodesiense- eastern and southern Africa, acute severe
* fexinidazole, pentamidine
* eflornithine, NECT, fexinidazole
Rhodesiense- eastern and southern Africa, acute severe
94
New cards
Human African Sleeping Sickness stages of sickness
1st (hemolymphatic stage) - weeks to months after infection, patient develops systemic illness w fever and swollen lymph nodes + trypanosomes present in blood stream (non-specific symptoms0 fever, headaches, lymph nodes, joint pain etc.)
\
2nd (meningoencephatic stage) -parasites invade CNS- brain + spinal fluid
* headache, mental dullness, poor cordination, sleeping (somnolence), disturb consciousness, coma, death
* **disrupts sleep cycle**
\
2nd (meningoencephatic stage) -parasites invade CNS- brain + spinal fluid
* headache, mental dullness, poor cordination, sleeping (somnolence), disturb consciousness, coma, death
* **disrupts sleep cycle**
95
New cards
How do trypansomas evade the immune system ?
Variable surface glycoproteins (VSGs) - alters VSGs in bouts of parasitemia in a chronic infecrtion
VSG switching is from genetic rearragenement
* VSGs stop complement binding
* Shed VSGs as decoy
* Produce TSIF - immunomodulatory factor
VSG switching is from genetic rearragenement
* VSGs stop complement binding
* Shed VSGs as decoy
* Produce TSIF - immunomodulatory factor
96
New cards
How to diagnose HAT?
Serological testing can be used for screening (only T.b. gambiense- cheap, simple, not 100% accurate) and/or checking clinical signs (swollen cervical glands)
\
Body fluids- find parasites (invasive, expertise required) lumbar puncture for staging (is it in CSF)
\
Make dx as early as possible before neurological stage
\
Body fluids- find parasites (invasive, expertise required) lumbar puncture for staging (is it in CSF)
\
Make dx as early as possible before neurological stage
97
New cards
Treatment of HAT
First stage- easier, less toxic + more effective
Gambiense = pentamidine (IM- usually well tolerated, 7-10d), fexinidazole (oral- 10d)
\
Rhodesiense = suramin (IV- 5-7d, causes urinary tract + allergic rxns)
\
Second- cross BBB, toxic + complicated
Gambiense = Eflornithine (IV 4x a day for 14d), NECT (oral + IV, 10d + 7d), fexindazole (oral- if not severe, 10d)
Rhodiense = melarsoprol (IV, 10d, drug resistance observed- can be fatal 3-10%)
\
Gambiense = pentamidine (IM- usually well tolerated, 7-10d), fexinidazole (oral- 10d)
\
Rhodesiense = suramin (IV- 5-7d, causes urinary tract + allergic rxns)
\
Second- cross BBB, toxic + complicated
Gambiense = Eflornithine (IV 4x a day for 14d), NECT (oral + IV, 10d + 7d), fexindazole (oral- if not severe, 10d)
Rhodiense = melarsoprol (IV, 10d, drug resistance observed- can be fatal 3-10%)
\
98
New cards
American trypanosomiasis overview
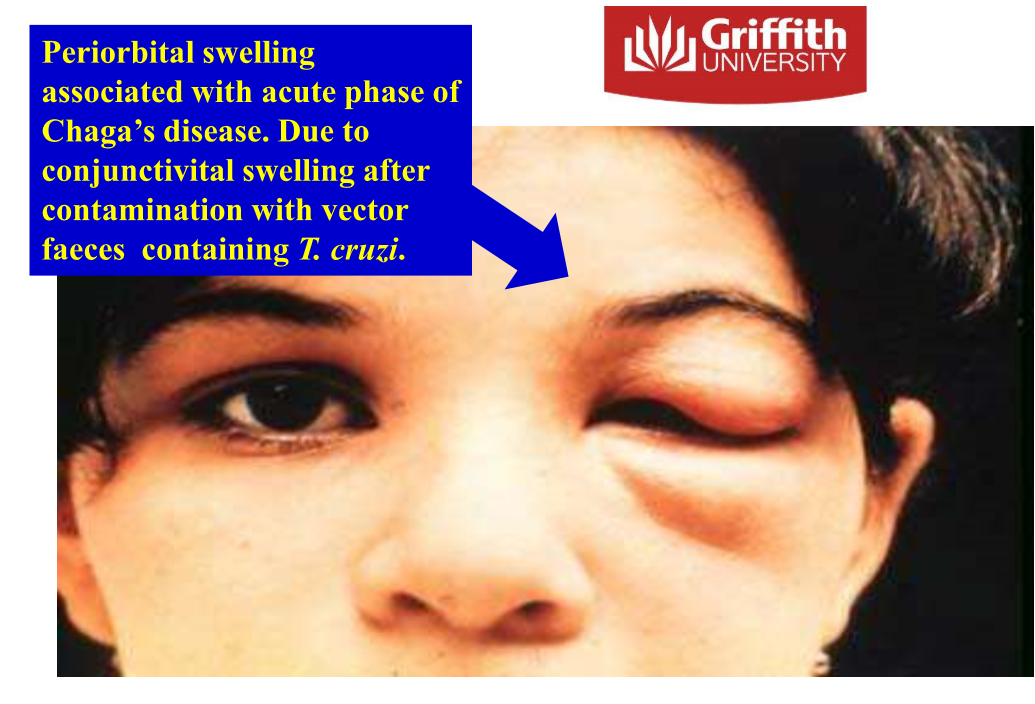
Chagas disease- Trypanosoma cruzi - zoonotic
Bloodstream and **intracellular** in many cells (muscles)
* trypomastigotes are extracellular
* amastigotes are intracellular
Transmitted via triatomine bugs (kissing bugs), also congenital transmission
Central and south america
\
Bloodstream and **intracellular** in many cells (muscles)
* trypomastigotes are extracellular
* amastigotes are intracellular
Transmitted via triatomine bugs (kissing bugs), also congenital transmission
Central and south america
\
99
New cards
Life cycle of american trypanosomiasis
Reduviid bug deposites faeces w trypomastigotes → wiped into body when it takes a blood meal → transforms into amastigote (ICF form) + invades tissue like muscle cells → cells
100
New cards
American trypanosomiasis phases of illness
Acute phase = 2 months after infection, mild or absent symptoms,