Topic 4: Haber process + Rate of attainment
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
24 Terms
What does the Haber process involve
it involves the reversible reaction between nitrogen and hydrogen to form ammonia gas
it is the industrial process used to produce ammonia gas
What type of reaction is the reaction between nitrogen and hydrogen which takes place in the Haber process
exothermic reaction (therefore the forwards reaction is exothermic)
Why are the gases hydrogen and nitrogen hard to react?
because nitrogen is very uncreative as it has a very strong triple bond
what is the process from which nitrogen is obtained?
fractional distillation
what is the raw material nitrogen comes from?
the air
What is the raw material hydrogen is from?
natural gases
What is the equation for the reaction between natural gas and steam to produce hydrogen?
methane + water vapoour → hydrigen + carbon dioxide
NH4 + H2O → 3H2 + CO2
What is the name of the area in the reactor where the main reaction to produce ammonia takes place
Convertor
What is the name of the catalyse used?
iron
Which temperature conditions would give the best yield of ammonia?
lower temperature, because the forward reaction is exothermic
Why are these temperature conditions (low temperatures) NOT used ?
Because the rate of reaction would be too slow
What pressure is used in the Haber process?
200 atmospheres
What temperature is used in the Haber process?
450 degrees
What 3 things come out of the converter in the Haber process?
hydrogen gas, nitrogen gas and ammonia gas - H2, N2, NH3
What happens to these gases when they come out?
they are cooled
What happens to ammonia after it is cooled?
it turns from a gas to a liquid (condenses) this is because it has a higher melting point than H2 and N2
What happens to the unreacted hydrogen and nitrogen in the converter?
they are recycled and are fed back into the converter
What conditions would give the best yield of ammonia ? why aren’t these conditions used?
low temperature
high pressure
low temperatures give a slow rate of reaction
high pressure is expensive to create and can be dangerous
What are the actual conditions that are used in the Haber process? and why aren’t the
a higher temp of 450 degrees
200 atmospheres
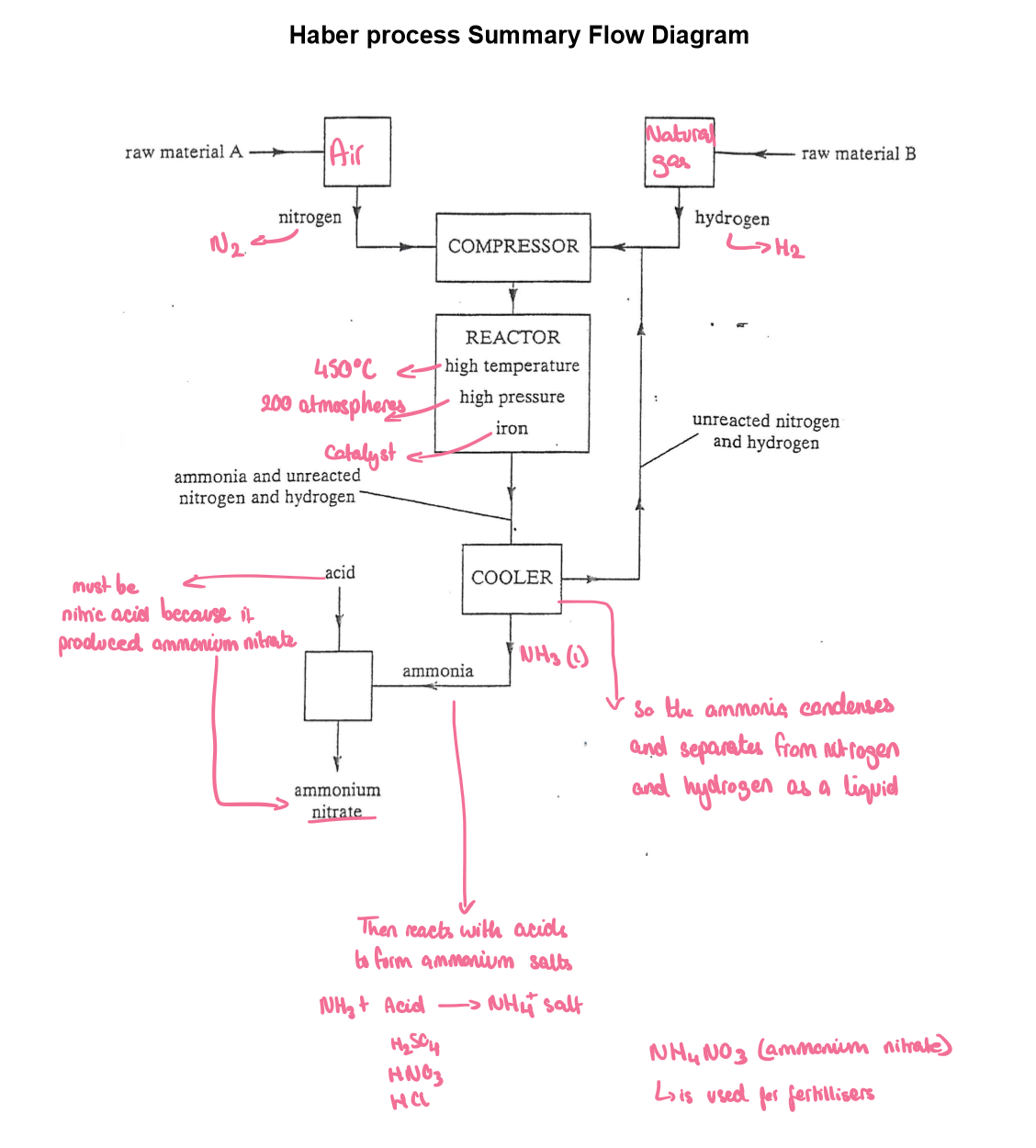
Here is a labelled diagram of the Haber process
What factors affect the rate of attainment of equilibrium?
temperature
Pressure/ Volume for gases and Concentration for aqueous solutions
Surface Area
catalyst
These factors affect the rate of the forwards and reverse reactions equally
What affect would any factor that increased the rate of the forwards reaction have on the reverse reaction?
it would increase the rate of the reverse reaction by the same amount and decrease the time taken to reahequilibrium
What affect would any factor that decreases the rate of the forwards reaction have on the reverse reaction?
it would decrease the rate of the reverse reaction by the same amount and increase the time taken to reach equilibrium
When a approaching questions about the rate of attainment use…?
the collision theory