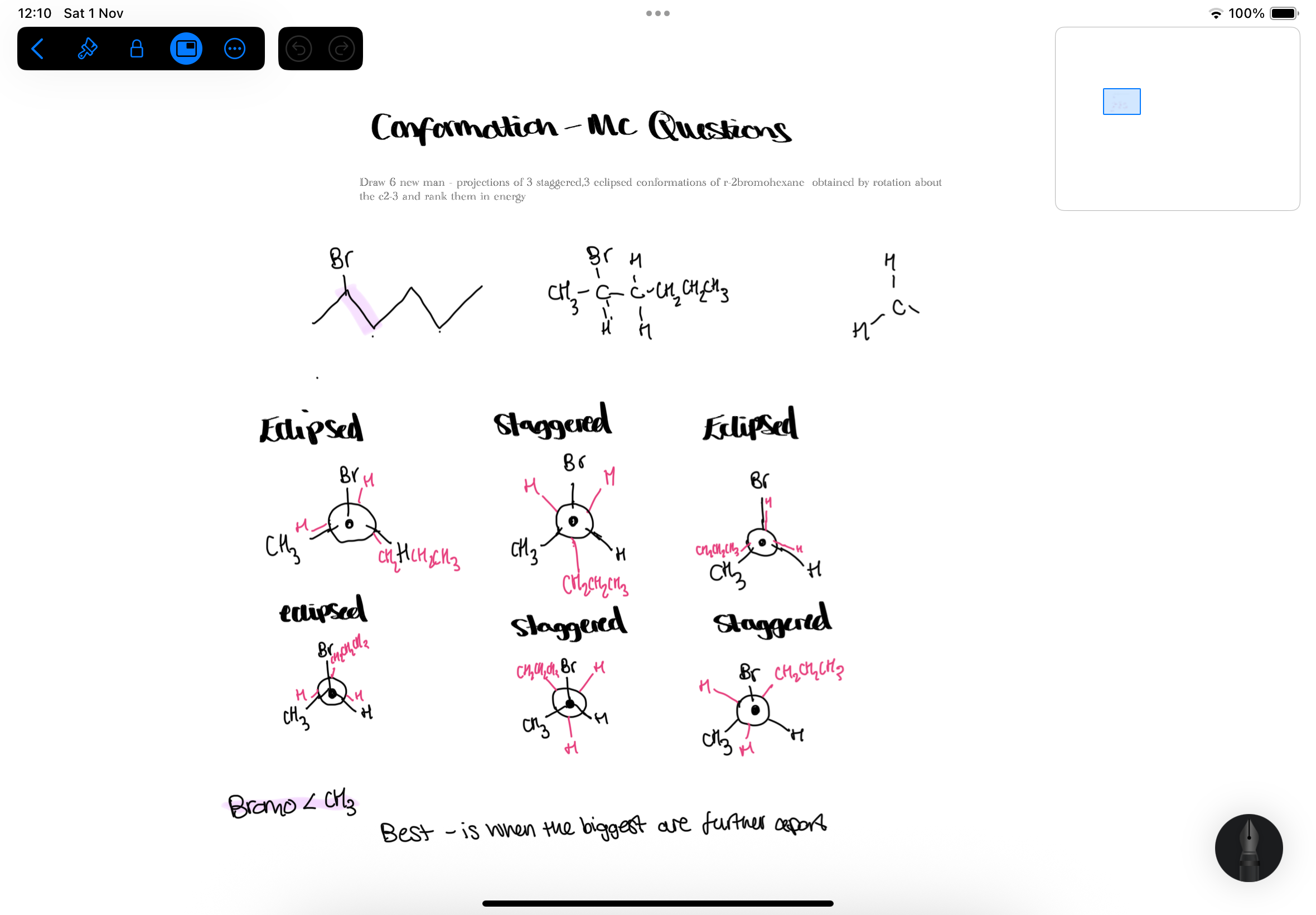
MC- molecular shape : conformation
1/7
Earn XP
Description and Tags
- eclipse, staggered graphs of energy
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
8 Terms
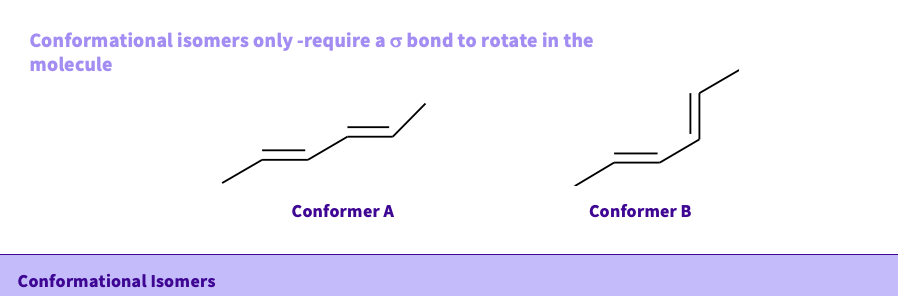
What is a Conformational isomer?
they only require a sigma bond to rotate in the molecule
NOTE- stereoisomers - are configurational isomers ( a bond has to be broken for the molecules to interconvert
Are the bond easy to rotate in conformational isomers?
energy is required to rotate a bond
this energy depends on the rest of the molecule eg. is there any conjugation
it is harder to rotate when sigma bonds when there are π bonds present
Why are rotations important in drugs ?
they are important for binding right the first time
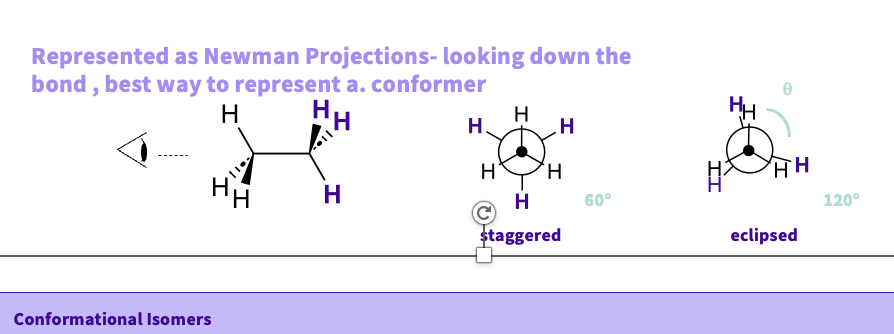
How are conformational isomers structured?
they can be staggered or eclipsed
staggered - not in line as far apart as possible ( less repulsion lower energy )
eclipsed- in line ( higher energy more repulsion )
How do we draw the different conformers of a molecule ?
use newman projections
looking at it from a straight down the bond
there is an infinite number of conformers as it rotates by one degree = new conformer ..
How do we rank the different conformers in energy?
eclipse- always the worst , Highest energy arrangement because the electrons clouds repel each other - not stable
staggered - best , lowest energy the electron clouds are the furthest apart so don’t repel - more stable
NOTE - highest energy when the biggest molecules are closest together
lowest energy when the biggest molecules ar ethe furthest apart
How do graphs represent energy of conformers ?
each point is a conformation
Can have some conformers that are identical in energy - because they have 2 of the same substituents
syn periplanar - global maximum- highest energy ( eclipse)
antiperiplanar global minimum- lowest energy `( staggered) \also have local maximum, local minimum
What affects the energy of a conformation?
when substituents are hydrogens - general rules:
-Staggered conformations always have lower energies
-Repulsion occurs between the electrons in the bonds
-The repulsion is greater when the bonds are aligned (eclipsed conformations) than when there is a larger dihedral angle between the bonds (staggered conformation)
-Each arrangement or conformation has a slightly different energy and stability.
-If there are no other factors the molecule will preferentially sit in the lowest energy staggered conformation.
when the substituents are larger:
-Steric (size) interactions begin to matter
-Some staggered conformations will be of relatively higher energy (lower stability)
-Some eclipsed conformations will be of relatively lower energy (higher stability)