Ch. 2 Water & Aqueous Solutions
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Water
Medium of life
Life evolved in water
Organisms typically contain 70-90% of water
Chemical reactions occur in aqueous solution
Water is a critical determinant of the structure and function of biological macromolecules
Water Molecules & Hydrogen Bonds
Sp3 oxygen (two bonds and two lone pairs)
104.5* bond angle
Net dipole moment
Can serve as both a H-bond donor and acceptor
Typically 4-10 kJ/mol
Strongest when oriented to maximize the electrostatic interaction
Ideally the three atoms involved are in a line (180*)
Hydrogen Bonds
Between the hydroxyl group of an alcohol and water
Between the carbonyl group of a ketone and water
Between peptide groups in polypeptides
Between complementary bases of DNA
Hydrogen Bonding in Water
Up to four H-bonds per water molecule gives water its
Anomalously high boiling point
Anomalously high melting point
Unusually large surface tension
Hydrogen bonds between neighboring molecules are weak (20 kJ/mol) relative to the H-O covalent bonds (420 kJ/mol)
3.4 H-bonds per water molecule in liquid
Ice: Water in a Solid State
Water has many different crystal forms, hexagonal ice is the most common
Hexagonal ice forms a regular lattice, and thus has a low entropy
Hexagonal ice contains more hydrogen bonds / water molecule
Ice has lower density than liquid water, therefore ice floats
Water as a Solvent
Water is a good solvent for charged and polar substances
Amino acids and peptides
Small alcohols
Carbohydrates
Water is a poor solvent for nonpolar substances
Nonpolar gases
Aromatic moieties
Aliphatic chains
The Hydrophobic Effect
Refers to the association or folding of nonpolar molecules in the aqueous solution
Is one of the main factors behind:
Protein folding
Protein-protein association
Formation of lipid bilayers and micelles
Binding of steroid hormones to their receptors
Does NOT refer to attractive direct force between two nonpolar molecules
Entropy and Low Solubility
Bulk water has little order and high entropy
Water near a hydrophobic solute is highly ordered and low entropy
Low entropy is thermodynamically unfavorable, thus hydrophobic solutes have low solubility
Lipids and Hydrophobic Effect
If lipid molecules disperse in water, nonpolar tail of each lipid molecule is surrounded by ordered water molecules (entropy of the system decreases)
If nonpolar portions of the molecule aggregate, fewer water molecules are ordered (released water molecules are more random, entropy increases)
Polar “head” groups H-Bond to water
Micelles maximize entropy by excluding the most water molecules
Ligand Binding & Hydrophobic Effect
Binding sites in enzymes and receptors are often hydrophobic
Such sites can bind hydrophobic substrates and ligands such as steroid hormones
Many drugs are designed to take advantage of the hydrophobic effect
Water Dissolving Salt
Polar water molecules surround ions to dissolve salts
Strong electrostatic interactions between the solvated ions and water molecules lower the energy of the system
Entropy increases as ordered crystal lattice is dissolved
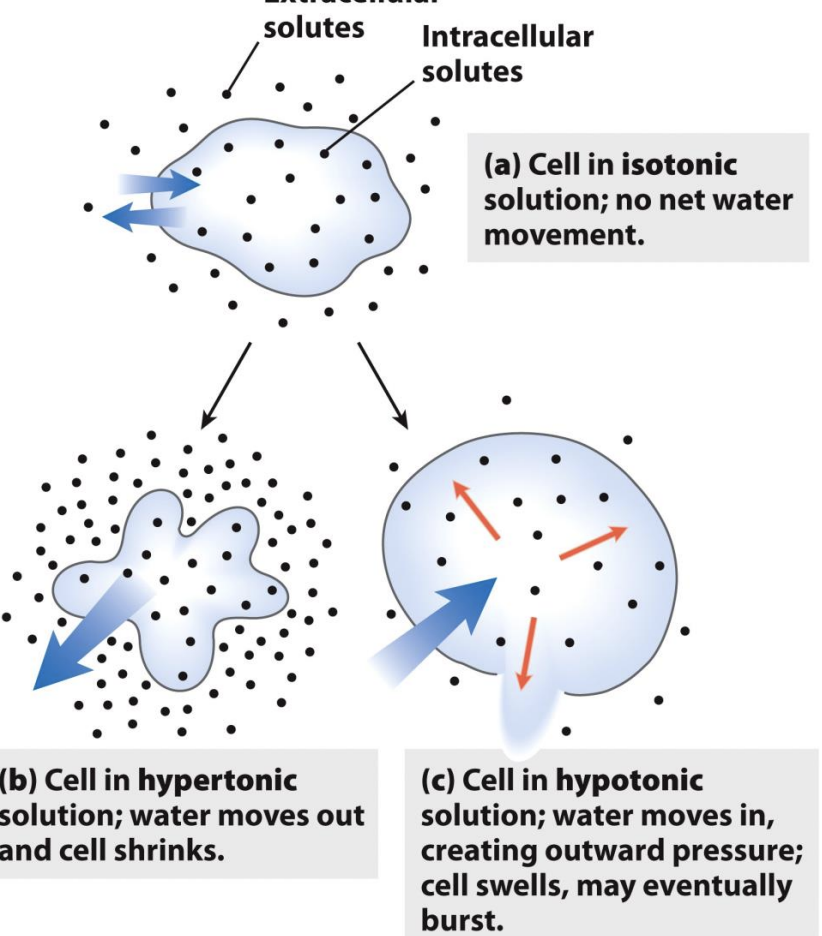
Extracellular Osmolarity
Water moves down its own concentration gradient from high conc. to low con.
More solute = less water
Cells in dilute salt solution are prone to bursting due to all the water moving from high (outside) to low (inside) concentration
Cells are concentrated bags of solutes!
Ionization of Water

• O-H bonds are polar and can dissociate heterolytically
• Products are a proton (H+ ) and a hydroxide ion (OH– )
• Dissociation of water is a rapid reversible process
• Most water molecules remain un-ionized, thus pure water has very low electrical conductivity (resistance: 18 M•cm)
• The equilibrium is strongly to the left
• Extent of dissociation depends on the temperature
Proton Hydration
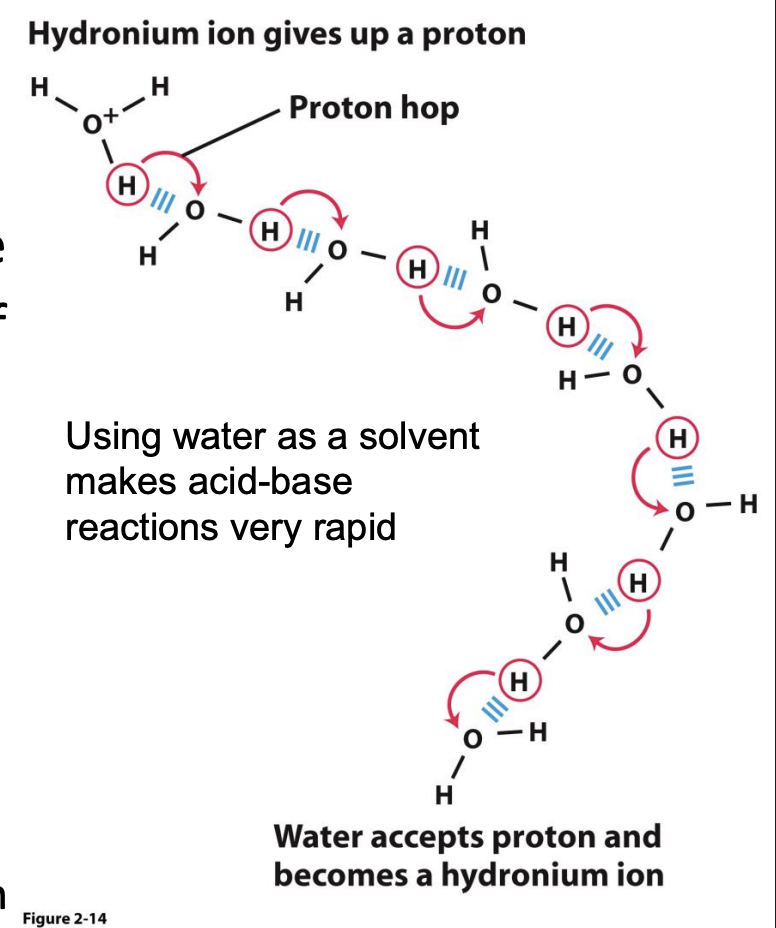
H+ does not exist free in solution. – They are hydrated to form hydronium ions (H3O+ )
• A hydronium ion is a water molecule with a proton associated with one of the non-bonding electron pairs.
• Hydronium ions are solvated by nearby water molecules
• The covalent and hydrogen bonds are interchangeable. – This allows for an extremely fast mobility of protons in water via “proton hopping.”