2: Water, Acids, Bases and Buffers Major Body Components
1/86
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
87 Terms
Water Characteristics: How Many Molecules can it Bind to?
3-4
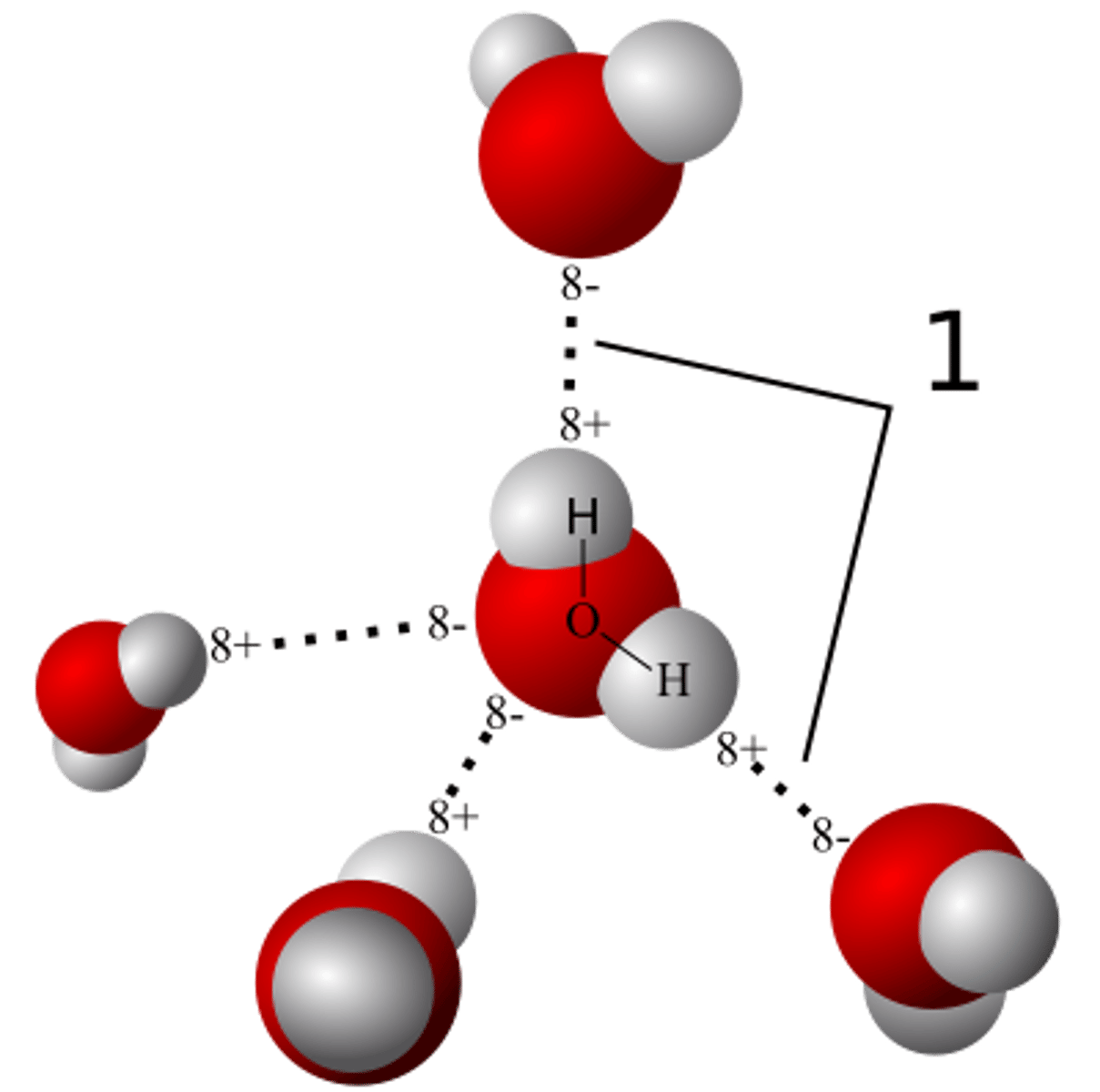
Bicarb/HCO3- in ECF or ICF? and why?
(ECF) acts as a major buffer in the blood and helps regulate blood pH, so it is more concentrated in the ECF where it plays a key role in maintaining acid-base balance.
Phosphatidyl choline
Phosphatidyl choline (lecithin) helps by acting as an emulsifier. It stabilizes fat droplets in water increase the surface area for digestive enzymes to break down dietary fats more efficiently.
Lipases
To digest these fats, they need to be broken down into smaller droplets. As fat droplets get smaller, their surface area increases, allowing lipid-digesting enzymes (lipases) to access and break down more of the fat.
Ion product of Water is Related to what Property of Water?
Self-Ionization into hydroxyl/hydrogen ions
Kw
1.0^-14 and H⁺/OH⁻ concentration is 1.0^-7 mol/L at pH = 7
H+ and OH- concentrations should add up to what?
1*10^-14
Buffers are ____ acids
weak
A weak acid can ___ or ___ a proton
donate or accept
An Acid Donates A Proton Rxn (water accepts protons from acids)
H₂O + HA ↔ H₃O⁺ + A⁻
Sulfuric Acid Rxn (what is it classified as)
B-hydroxybutyric acid (what is it classified as ) rxn
Acetoacetic acid (what is it classified as) rxn
Strong Acid ; H₂SO4 --> 2H⁺ + SO4(2⁻)
KB; gives up its H from COOH in KB ↔ (KB)with COO⁻ + H⁺
CB: B-hydroxybutyrate
KB; does the same as above KB
CB: acetoacetate
pka indicates
the willingness of acid to donate a proton
pKa of sulfuric acid - completely disassociated, COOH is 3.8 and Ammonium is 9.25
Water donates protons to bases
H₂O + B (ammonium NH3) ↔ BH⁺ (NH4+) + OH⁻
Ammonium at physiological pH tendencies
HH Equation
pH = pka + log (CB/A)
pH = pKa @ 50% diassociation where [A-] = [HA]
pka - strong acid has a low pka and high Ka
Ka = [H+][CB or A-] / [HA} with more being on top if SA
Buffer Resist Change (OH- added)
for +1 pKa: Acetate (removing protons) drops off protons from COOH to make water (from OH+H) to resist a rise in pH.
Acetic acid is all found in the acetate form at one above pKa so no more H+ to give to OH- so no more buffer
Typically: HA --> H+ + A- & OH- + H+ --> H2O keeps Ka constant
Buffer Resist Change (OH- removed or acid added)
for -1 pKa: reducing OH- ions trying to lower pH but acetic acid counteracts that by sucking in H+ ions.
Typically: H+ + A- --> HA to keep Ka constant
At pH of 7.4, what form will acids be in?
Anion; 50-50 around 4.5: dotted line is 7.4 ish. Clear where its all CB in anion form.
At pH = 7.4 what form would ammonium (NH4+) be with pka of 9.25 and dihydrogen phosphate with pKa of 6.8
NH4+ --> NH3 + H+ (it would be in its ammonium NH4+ form)
Dihydrogen:monohydrogen phosphate: 50-50
Hemoglobin is what and functions where
minor buffer in RBC and plasma
Bicarbonate is what and functions where vs phosphate buffer system
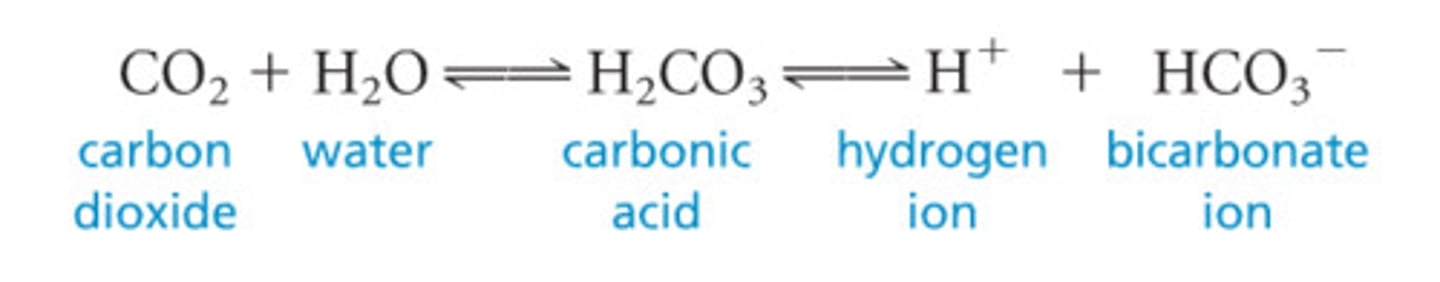
Its in CB form. Formula for carbonic acid: H2Co3. In intracellular fluid, you have phosphate buffer system which is major buffering system in ICF. H2PO4 can give up both protons but it only like to give up 1 proton and pKa for giving up one proton is 6.8.
Bicarbonate System
CO2: weak acid and buffered
Carbonic anyhdrase makes carbonic acid (H2Co3) from CO2+water.
Exhale Co2 so more bicarbs formed. pKa = 6.1 close to 7.4 good things.
At a pH of 7.4, still find mostly carbonic acid since more than 1 pH unit away from the normal physiological pH of 7.4. To get a pH of 7.4 with an apparent pKa value of carbonic acid, we have to have a ratio of 20 bicarbonates for every carbonic acid.
Metabolic Acidosis
Increased [H+] OR decreased [HCO3-]
Decreased pH
- Ketosis
- Kidney damage
- Chronic diarrhea
- Excessive lactate production —> tissue hypoexia
![<p>Increased [H+] OR decreased [HCO3-]<br>Decreased pH<br>- Ketosis<br>- Kidney damage <br>- Chronic diarrhea <br>- Excessive lactate production —> tissue hypoexia </p>](https://knowt-user-attachments.s3.amazonaws.com/a7bde2d4-bc2f-4ee6-9b08-1006dc685304.png)
Metabolic alkalosis
Decreased [H+] OR increased [HCO3-]
Increased pH
- Prolonged vomiting
- Excessive ingestion of antacids
![<p>Decreased [H+] OR increased [HCO3-]<br>Increased pH<br>- Prolonged vomiting<br>- Excessive ingestion of antacids </p>](https://knowt-user-attachments.s3.amazonaws.com/fde23456-5669-496a-b19f-4a95fc5cb552.png)
Carbonic anhydrase role in RBC, renal tubules, parietal cells, and pancreatic cells
Carbonic anhydrase imp in RBC, renal tubules (excretion/reabsorption of bicarbonate), parietal cells (stomach acid HCl formation by bicarb cells), pancreatic cells (bicarb to SI for a buffer of stomach acid)
Bohr curve (in relation to carbonic anyhdrase)
Carbonic anhydrase converts CO₂ + H₂O ⇌ H₂CO₃ ⇌ H⁺ + HCO₃⁻.
↑ CO₂ / ↑ H⁺ (acidic) → right shift → decreased hemoglobin affinity for O₂ → easier O₂ unloading in tissues.
↓ CO₂ / ↓ H⁺ (alkaline) → left shift → increased affinity for O₂ → harder to release O₂.
Temperature and 2,3-BPG also modulate the curve (↑ temp & ↑ 2,3-BPG = right shift).
Quick Memory:
Right shift = Release O₂ (think “exercise”)
Left shift = Love O₂ (hold onto it)
Red Blood Cell Mechanism (in relation to carbonic anyhdrase) "CO2 a weak acid"
High CA in RBC: CO₂ + H₂O → H₂CO₃ → H⁺ + HCO₃⁻
Hemoglobin buffers H⁺ (until it must bind O₂ → ↓ H⁺ buffering → right-shift O₂ curve).
Phosphate buffer exists but is weaker; overwhelmed → hemoglobin takes over.
CO₂ from metabolically active tissues enters RBC, converted by CA, HCO₃⁻ exchanged out to carry more H⁺.
Extra H⁺ can also come from ketone bodies or lactic acid
Hepatic Cell Mechanism
In hepatic cell, we have generic protein acting as a buffer to bind to protons and phosphate buffering system to make dihydrogen phosphate instead of mono.
Bicarb major buffer system in ECF. Proteins, phosphate buffer system inside cells. Hemoglobin/proteins/carbonic anhydrase/bicarb in RBC.
Physiological pH Range
Blood pH is slightly alkaline (7.3-7.4). Bc its slightly alkaline, when we say patient is in acidosis, it doesn't mean blood pH below 7. Its just below reference range. When a patient has alkalosis condition, the pH is above reference range.
Resp Acidosis
Increased H+ | PCO2 | HCO3-
Lower pH
- Pneumonia
- Emphysema
- Severe asthma
- Acute pulmonary
- Congestive heart failure
- Drug inhibition of respiratory center —> ethanol/barbiturates
Metabolic Alkalosis Cause
Decreased [H+] OR Increased [HCO3-] High pH | High PCO2.
Rare
- Prolonged vomiting
- Excessive ingestion of antacids
Resp Alkalosis
Decreased [H+] OR Decreased HCO3-, Decrease PCO2, Increased pH
- Hyperventilation
- High latitude leads to it
Patient case
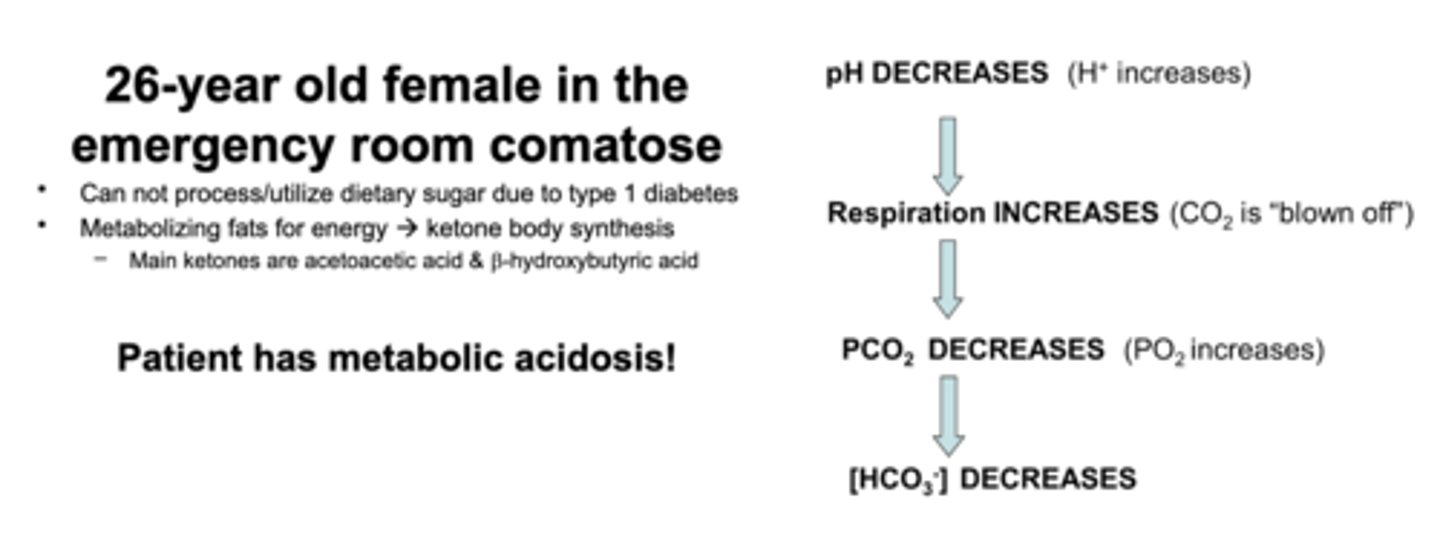
Type 1 Diabetes and KB - can't process dietary sugar due to type 1 diabetes so metabolizes fats for energy --> KB synthesis. Main ketones are acetoacetic acid and b-hydroxybutyric acid.
Choline
quaternary amine
Vitamin A (structure and function in night blindness)
11-cis retinal (aldehyde)
When light hits that cis double bond, that provides enough energy to isomerize into a trans double bond.
Cis → trans changes conformation, starts cascade, electrical impulse to brain → light.
Thats how eyes sense the presence/absence of light. Patients with Vitamin A deficiency have night blindness which is poor adaptation to adjustment of low light conditions
Retinoic Acid
Another form of vitamin A is Retinoic acid (all trans)
It ends with COOH group (not an aldehyde)
All double bonds in trans position not cis
This form of vitamin A functions (not a steroid hormone - similar) as steroid hormone transcription function ligand. Binds to transcription factor to reg expression of diff genes
Diff genes - vitamin binds to diff transcription factor that reg expression of other genes.
Vitamin A oxidation-reduction
Oxidation-reduction : alcohol retinol form → oxidize to aldehyde → oxidize it further to COOH
Sorbitol (glucitol) - glucose
Sorbitol is what builds up and causes cataracts, neuropathy, and retinopathy in patients with diabetes.
If we have sm glucose around for so long, an alternative metabolic pathway for glucose is to make sorbitol.
Great for dealing with glucose but next rxn that takes sorbitol to fructose is slowed.
Sorbitol: a) builds up | b) act as a solute | c) put water in tissues with eyes and causes retinopathy/cataracts/neuropathy —> cells to die.
Alt pathway for glucose and normal pathway to make fructose - patients with diabetes with sm glucose - alt pathway to make sorbitol n sorbitol rxn slow so sorbitol hangs for too long.
Used as a sugar substitute, laxative (hyperkalemia): acts as a laxative because it is not fully absorbed in the intestine. When consumed in excess, it remains in the gut, where it draws water into the intestines through osmosis. This increased water content helps soften stools and stimulates bowel movements, leading to its laxative effect
galactitol (dulcitol) - galactose
Refers to patients with galactosemia - can not metabolize galactose
Galactose + glucose = lactose —> excess galactose when drinking milk
Excess galactose —> reduced to galacitiol
Galactitol can not be metabolize further → builds up in eye and causes pulls water in lens of eye and causes cataract in 18 month patients since milk primary food source
Mannitol (mannose)
Mannitol used as osmotic diuretic —sugar alcohol that is not metabolized
pulling water to reduce pressure in eyes glaucoma and cranium intercranial pressure
Fructose
ketone sugar
Glucose and galactose
aldose sugar (aldehyde)
Retinol/aldehyde/sorbitol/galcitol oxidation vs fructitol
Retinol oxidized to retionol/retinaldehyde being further oxidized to retinoic acid COOH form
IF we oxidize aldose sugars, we get carboxylic acid COOH
IF we reduce aldehyde, we get an alcohol (sorbitol/galacitiol)
Sugar alcohols like sorbitol or galictol - not a fructitol —>
Not able to reduce ketone to alcohol. The aldehyde reduced to aldehyde and get sugar alcohol.
aldose reductase
aldehydes reduced back to alcohol is by aldose reductase enzyme that takes aldehyde sugars and makes alcohol

Glucose to glucuronic acid
oxidation to make fat soluble --> water soluble molecules (usually 2 of them added).
Put it on bilirubin (2 glucuronate molecules on it) to make it more soluble.
To get rid of Vitamin A, conjugate to glucuronic acid and secrete it in urine.
Get rid of vitamin A
conjugate to glucuronic acid and excrete in urine
Additions to sugars
Phosphate addition (glucose-6-phosphate) - traps glucose in cell if phosphorylated!!
Ring Structures are _____. Alpha - Beta?
Carb found as ring structure. Hydroxl group on first carbon dictates whether its an alpha or beta sugar.
If OH below, alpha sugar. If above, beta sugar.
Lactose linkage is ______
beta 1,4 : galactose + glucose
Lactase as u get older
it goes down
alpha 1,4 type of point?
linear straight line of glucose molecules for starch
alpha 1,6 type of point?
branch point for starch
Starch and glycogen have what points?
branch points and eventually linear string for new bond
Glycogen structure
alpha 1,4 linear and every so often alpha 1,6 : its structure itself is feathery.
Glycogen in which 2 body areas? What does one have that the other doesn't?
Stored in liver and muscle.
Liver has glucose-6-phosphatase → removes phosphate → releases glucose into blood to maintain blood glucose.
Muscle lacks this enzyme → keeps glucose-6-phosphate for its own metabolism.
Starch (2 types)
amylose linear (1,4) chain and amylopectin branched (1,6)
First enzyme that breaks down carb?
salivary amylase (enzyme to break down amylose is amylase)
Cellulose structure
beta 1,4 : linear string of glucose molecules
Amylase can not break down beta 1,4
Cellulose indigestible by human enzymes. Functions as a dietary fiber.
Sucrase, amylase, maltase, isomaltase vs lactose :
all break alpha 1,4 except lactose breaks beta 1,4
isomaltase: alpha 1,6
Dietary vs Function Fiber
nondigestable carb and lignin intrinsic/intact in plants
that intrinsic plant molecule - isolated, extracted or manfactured nondigestable carbs that have physiological beneficial effects on humans
Example: psyllium (Metamucil) for functional fiber (includes dietary component)
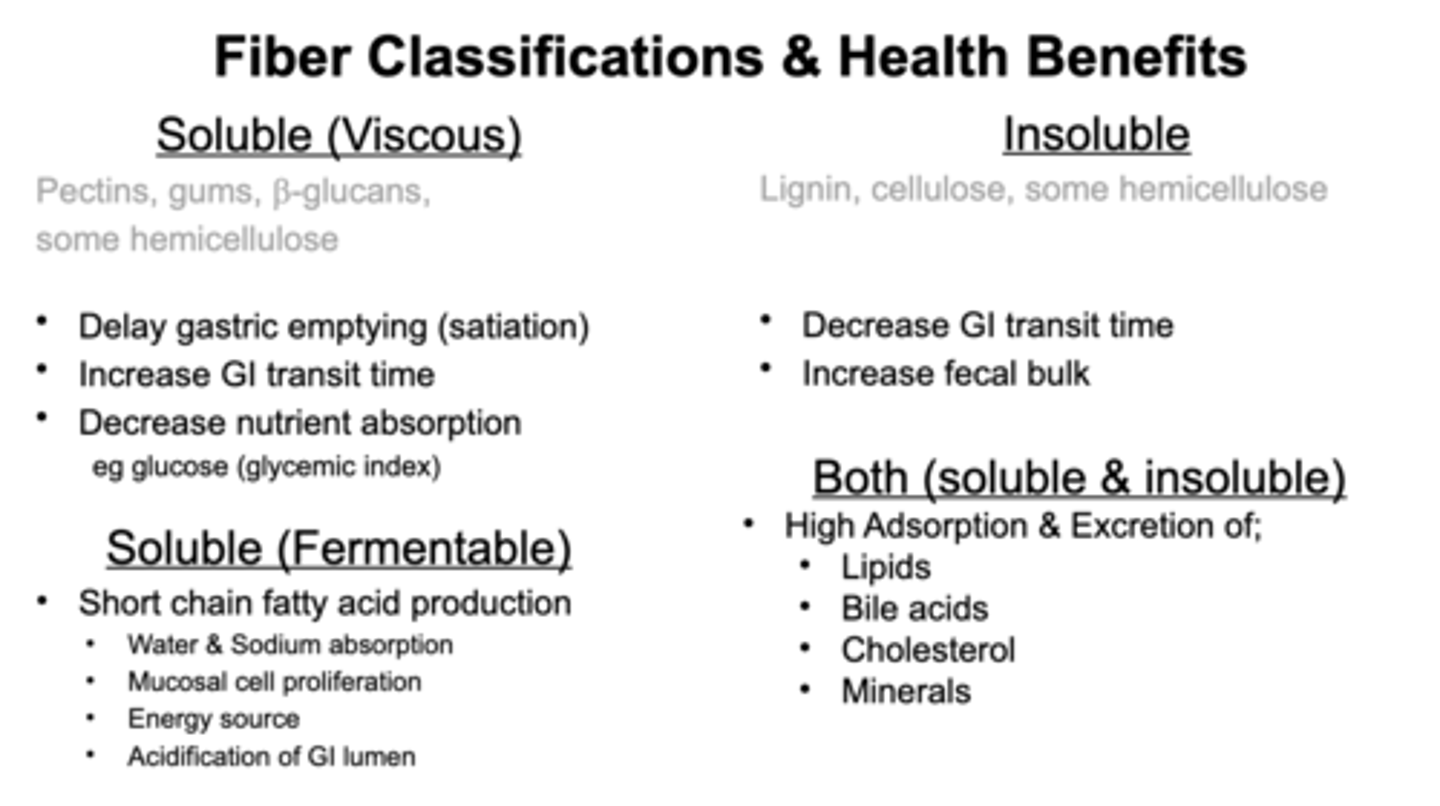
Fibers types w/ pectin example
Fibers property = diff functionalities. Groupable based on solubility, viscocity, and fermentability.
Pectins: stickly-like substances (high viscocity)
Solubility - soluble or come out of solution
Fermentability - can colon microbes metabolize it?
High vs low soluble, viscous, fermentability - for fibers
Some of our fibers low soluble, low viscous, low fermentability increase transit time through GI tract. They bulk up the stool.
Ones in middle (medium, fermentability, medium viscosity) increase transit time n pull water in stool making it softer.
Highly viscous, fermentable: increase nutrient bioavailability and will create short chain FA due to fermentability. Soluble, non-viscous - prebiotic/probiotic food like immulin, GOS
BOTH: High absorption and excretion —> lipids, bile acids, cholesterol, minerals
FIber Health Benefits
affect nutrient absorption, glycemic index
(more fiber, lower glycemic index as it slows rate of digestion - good thing for Type II diabetes and makes them feel full longer - increasing satiety to consume less food) - soluble fibers
Cheerios - fibers
Good for health. Has fibers that bind cholesterol and help its excretion
It does not help ppl lower cholesterol. It does help excrete cholesterol but patients already making so much.
FODMAP diet
Not a thing as non-celiac gluten sensitivity. They are either celiac or not.
FODMAP responsible for non-celiac gluten sensitivity (fermentable oligo, di, monosaccharides, and polyols).
Colon microbiome can ferment them.
Shorter sugars are monosaccharides. Most problematic disaccharide is lactose. (became of lactase resistance and lactose intolerance).
High consumption of FODMAP - GI distress.
FODMAPS
has to be short duration and under supervision of dietician (1-2 weeks n then add FODMAPS back in). Their in food, grains, candies (Sugar alcohols) etc.
Low FODMAP diet for patients with IBS to get improvement in symptoms.
Lipids
everything hydrophobic is a lipid
Lipids can be FA or steroids (cholesterol based).
Break down FA into triglycerides (storage)
phosphoglycerides (phosphate head group to 2 fatty acid tails), and sphingolipids.
Sphingolipids have sphingosine backbone (not glycerol backbone). Sphingosine is serine-palmatine (serine + 2 fatty acids). Phosphohead group (phosphatidyl choline) = sphingomyelins. If you put sugars, you get glycolipids.
FA
FA can be short-chain FA.
short-chain FA are all saturated FA. Not enough carbons to have double bonds.
Fermentable Fiber Metabolism Creates Volatile Short Chain FA Absorbed by Humans
Short chain FA can be branched like methyl group popping off one of the carbons.
Short chain FA 2-5 carbons in length and include acetic acid, propionic acid, butyric acid, and valeric acid.
Short chain FA in fiber - bacteria metabolizing fibers into short chain FA and directly absorbed into the bloodstream to liver to tissues for systemic effect.
Colon cells like butyrate. Bacteria making butyrate - short chain FA from fiber metabolism. Butyrate feeds your own colon epithelium cells.
Short & Medium Chain FA Absorbed Directly to Portal Blood
Short chain/medium FA --> bloodstream --> liver.
6-12 carbons in length: mostly saturated or monounsaturated.
Odd chains like 7 carbons/9 possible. We can have branch points of medium chain FA.
The reason we only list 2 carbons is cuz acetyl-coA is 2 carbons and how we metabolize break down FA is removing 2 carbons at a time: acetyl-coA.
Once we have odd chain FA, we remove 2 carbons until we left with 3 and then we have priopionic acid (proprionate) --> TCA cycle and acetyl-coA.
Proprioniate - can't remove 2 carbons and leave 1 carbon so leave it and enters TCA cycle.
Long Chains
12 carbons to 20 carbons in length. Talking abt myristic, palmitic, stearic, arachidic acid.
long chains greater than 22 carbons: behenic, lignoceric, certocic acid, arachidonic acid.
Arachidonic acid is an omega-6 fatty acid found in fish oil, which is rich in omega-3 fatty acids.
We can have unsaturated, polyunsaturated, monounsaturated FA. Branch points FA. odd carbon length fatty acids, and branched.
Naming Fatty Acids
Omega first double bond from methyl end
Delta describes each double bond from COOH end
Carbon # and number of double bonds: 18 : 3 which is 18 carbons and 3 double bonds
2 Essential FA Drive Long-Chain Polyunsaturated Acids
2 essential FA:
Linoleic acid (n-6, 18C, 2 double bonds)
Alpha-linolenic acid (n-3, 18C, 3 double bonds)
Humans can’t synthesize beyond 16C → must get 18C FA from diet.
Both share same enzymes:
Δ6-desaturase (adds double bond)
Elongase (adds 2 carbons).
Compete for enzymes:
n-6 → arachidonic acid (pro-inflammatory).
n-3 → EPA, DHA (anti-inflammatory, brain/heart health).
Diet tip: Eat fish 2–3x/week or supplement fish oil; avoid excess n-6 oils
n3 LC-PUFA in Health & Disease
More about omega 3, 6 FA.
Historically, we had a good ratio of 1N6: 1N3 with very little saturated fat. Total fat not bad but low compared to today.
Started industrializing food supply, FA went up. Blip of trans fatty acids that came back down. Oils contain mainly n6. Fish oil dropped a little. Now the U.S. consumes 20N6 : 1N3.
General consensus: 3-4N6:1N3 lowers risk of disease
Drug of EPA and DHA. Specifically ethyl esters that add another group to them but its rly just fish oil. Prescribe drug to treat hypertriglyceridemia. Elevated blood triglycerides go down.
Trans Double Bonds are Rarely Found in 'Natural' Fatty Acids
Trans fats and trans double bond. Fatty acids in nature: usually have cis-double bonds. Trans double bonds from processing fat/fat foods. Hydrogenated fats to make liquid fats solid at room temp like margenine (hydrogenated so now solid at room temp), oil, and other fats.
Clinically trans fat
They lower HDL (good cholesterol)
raise LDL (bad cholesterol)
increase TG/inflammation, and basically heart attack.
Its required to be listed on food labels so ppl avoid it.
Fatty Acids are Metabolized for Energy OR Metabolized for Lipid Mediator Generation
Fatty acids are important for energy and for generating lipid mediators.
N6:N3 ratio matters because dietary FA are incorporated into phospholipid membranes.
If you eat mostly N6, your membranes contain mostly N6 → mainly arachidonic acid → metabolized to proinflammatory mediators (prostaglandins, leukotrienes, thromboxanes).
Low DHA/N3 → fewer anti-inflammatory mediators produced.
Note: Not all N6 are proinflammatory, and not all N3 are anti-inflammatory; these are general trends, not absolutes.
Fatty Acid + Glycerol = Acylglycerol
Triglycerides: glycerol backbone + 3 FAs (any chain/unsaturation/branch). Main energy storage form of acetyl-CoA.
Removing 1 FA → free glycerol carbon can accept a phosphate head group → phospholipid.
Phospholipid head groups: phosphatidylcholine (lecithin), phosphatidylserine, phosphatidylethanolamine, phosphatidylinositol (signaling).
Phosphatidylserine: inner leaflet of membrane → flips to outer leaflet during apoptosis.
All phospholipids: act as emulsifiers/micelles (heads out, tails in).
Lecithin: used in food/protein powders to emulsify fats.
Just know phosphatidylinositol (seen in signaling) - no details needed.
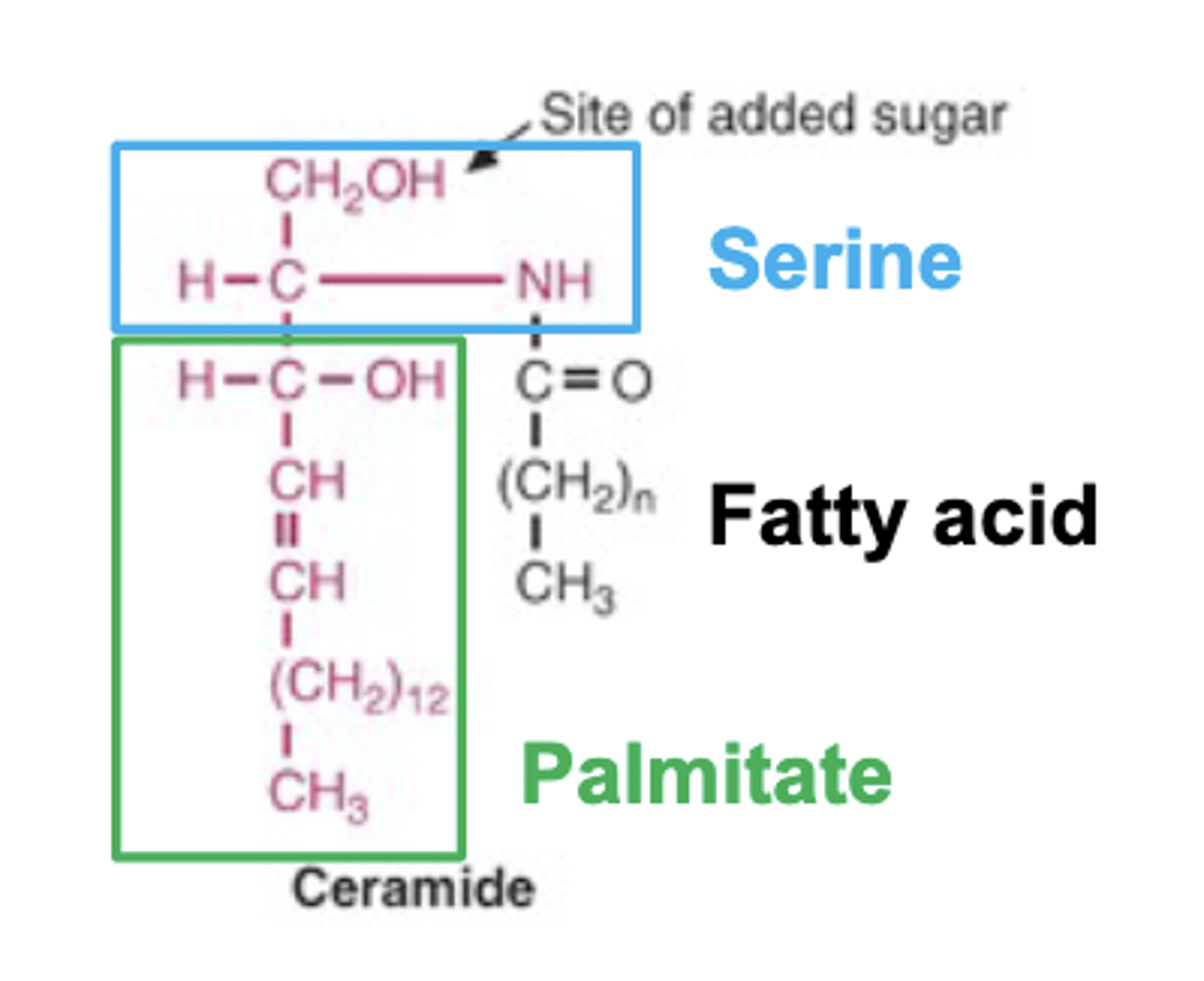
Ceramide
Structure: Serine + Palmitate = sphingosine backbone, then attach 1 fatty acid (any chain/unsaturation) to the nitrogen → ceramide.
Free hydroxyl group on serine can accept:
Phosphate head group → phosphosphingolipids
Carbohydrates → glycosphingolipids
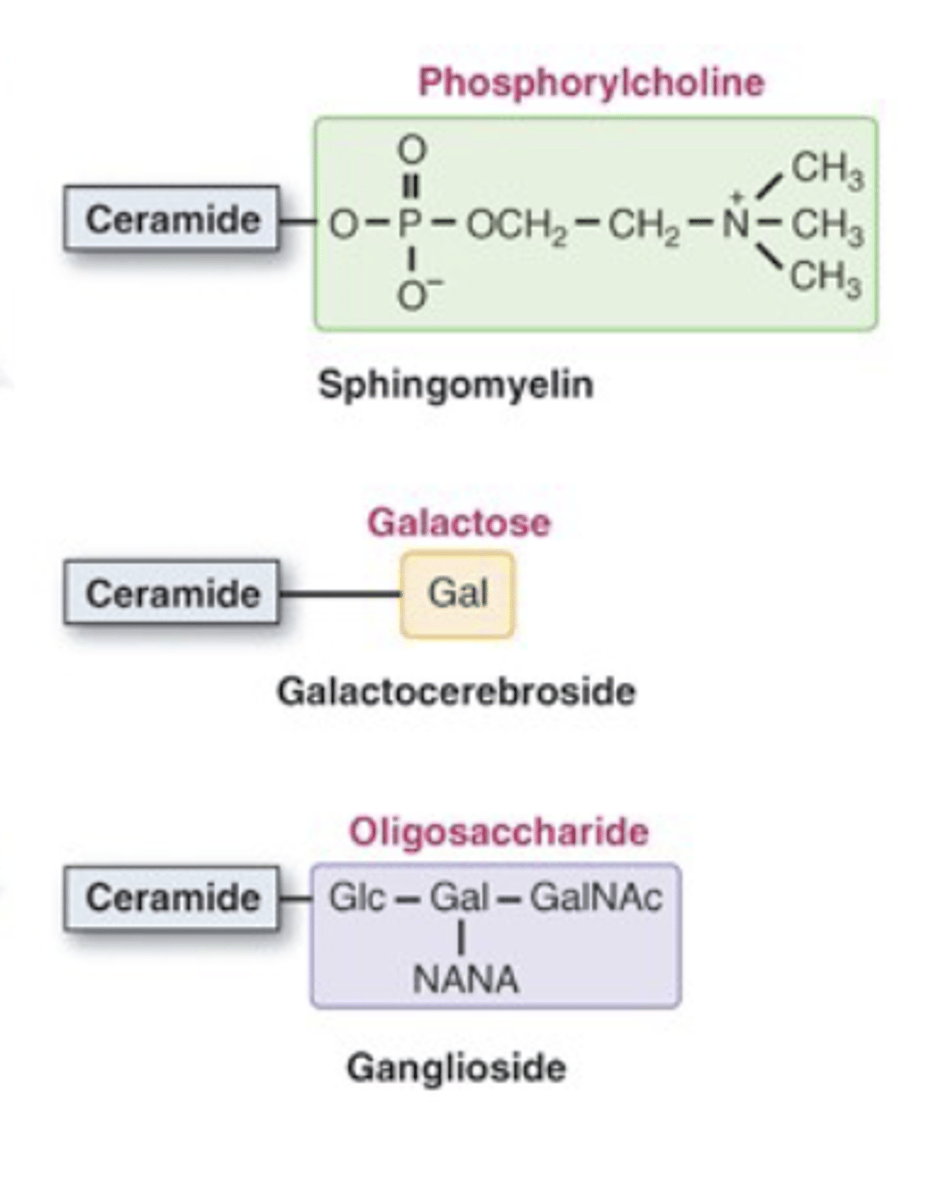
Ceramide + Phospho-Head Group = Sphingolipids Ceramide + Sugar = Glycolipids
Sphingomyelin = ceramide + phosphate head group; found in all plasma membranes (not specific to myelin).
Glycolipids = ceramide + sugar; enriched in nervous tissue (e.g., galactocerebrosides).
Glycolipid subtypes:
Cerebrosides: single sugar
Gangliosides: sugar + sialic acid (neuraminic acid / NANA)
Neuraminidase cleaves sialic acid from gangliosides.
Fatty Acid Melting Temp Important in Membrane Fluidity
Saturated, long-chain FA → straight, tight packing → ↑ melting temp → more viscous/stiff membranes.
Unsaturated FA (cis double bond) → kinks, loose packing → ↓ melting temp → more fluid membranes.
Lipid rafts = viscous microdomains enriched in cholesterol, sphingolipids, and long-chain saturated FA
Steroids & Cholesterol-Based Lipids
Steroids = lipids with cholesterol-based ring structure (multiple fused rings).
Cholesterol: steroid + OH group (steroil) → found in membranes, precursor for hormones & bile acids.
Biosynthesis: body makes enough cholesterol; dietary intake not required.
Steroid ring structures are present in:
-Bile acids (e.g., cholic acid)
-Hormones (estradiol, testosterone, aldosterone, cortisol)
-Vitamin D
Cholesterol is the precursor for steroid hormones and bile acids (which act as emulsifiers).
Generic Steroid Hormone Mechanism of Action; Genomic & Non-Genomic Actions
Main function: Most steroid hormones act as ligand-activated transcription factors to change gene expression. Some also have non-genomic actions.
Transport in blood: Lipid-soluble → need binding proteins for stability in aqueous environment.
Cell entry: Diffuse through lipid membranes. Inside, may bind intracellular receptors.
Genomic mechanism example (cortisol):
Cortisol binds cortisol receptor → homodimerizes → translocates to nucleus → binds hormone response elements (HREs) → alters gene transcription → increases glucocorticoid response.
Vitamin D example:
Vitamin D binds carrier protein in blood → enters target cell → binds vitamin D receptor → forms transcription complex → binds vitamin D response element (VDRE) on DNA → recruits RNA polymerase → increases gene transcription.
HREs: Specific DNA elements for hormone-receptor complexes; e.g., VDRE for vitamin D, RARE for retinoic acid.
Structure/Function of Fat-Soluble Vitamins (Vitamins A & D vs E & K)
Vitamins A & D:
Function as ligands for steroid hormone transcription factors.
Vitamin A: also critical for vision.
Vitamin D: has steroid ring structure, derived from cholesterol precursor (but making cholesterol doesn’t guarantee vitamin D).
Only A & D have this steroid-like transcriptional function.
Vitamin D requirement: ~15 min sun exposure on hands/face in summer can meet needs.
Other fat-soluble vitamins (non-steroid functions):
Vitamin K → cofactor for carboxylation reactions.
Vitamin E → functions as antioxidant.
What forms of vitamin A function in the eye? In other tissues?
Eye (vision): Vitamin A aldehyde form (retinal / cis-retinal) participates in the visual cycle.
Other tissues: Vitamin A as retinoic acid (trans-retinoic acid) acts as a ligand for steroid hormone-like transcription factors.
Excretion: Vitamin A/metabolites can be conjugated with glucuronic acid (glucuronidation) → increases water solubility for excretion.
Vitamin D Can Be Synthesized by Humans & Must be Activated to Function
Precursor: 7-dehydrocholesterol (from cholesterol) in skin.
Sunlight exposure → converts 7-dehydrocholesterol → vitamin D3 (cholecalciferol).
Activation: Vitamin D3 must be hydroxylated (liver & kidney) to become functional.
Sun exposure needed: ~10–15 min on hands/face sufficient for most people.
Factors affecting synthesis: skin pigmentation (darker skin → more time), sun angle/latitude
What is the likely cause of his bowed tibiae?
Vitamin D deficiency in childhood → impaired calcium homeostasis → rickets (soft bones → bowed legs).
Vitamin D-regulated genes:
Calbindin D9k → intestinal calcium absorption
Calbindin D28k → renal calcium reabsorption
Vitamin D is essential for regulating calcium homeostasis via these genes in intestine and kidney
Acid-Base Mnemonic
Respiratory Opposite: pH change is opposite PCO2
Metabolic Equal: pH change is the same as bicarbonate
26-year old female in the emergency room for comatose
1. pH decreases —> H+ conc goes up
2. Respiration increases —> CO2 blown off
3. PCO2 decreases —> PO2 increases
4. [HCO3-] decreases