Reactivity series
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
How can metals be arranged in a reactivity series based on their reactions with:
water
dilute hydrochloric or sulfuric acid?
Some metals are more reactive than others.
The order of reactivity can be determined by adding acid to different metals and observing the rate of reaction.
For example, when hydrochloric acid is added to iron (Fe) then bubbles of hydrogen are produced slowly. However, if the same acid is added to zinc (Zn) then bubbles will be produced more quickly. This tells us that zinc is more reactive than iron.
Instead of using acid, water can be used to test the relative reactivity of metals. However, many metals are too low in the reactivity series to react with water, so if they do react they have a high reactivity.
How can metals can be arranged in a reactivity series based on their
displacement reactions between:
• metals and metal oxides
• metals and aqueous solutions of metal salts.
You can see if one metal is more reactive than another by using displacement reactions:
Easily seen when a salt of the less reactive metal is in the solution
More reactive metal gradually disappears as it forms a solution
Less reactive metal coats the surface of the more reactive metal
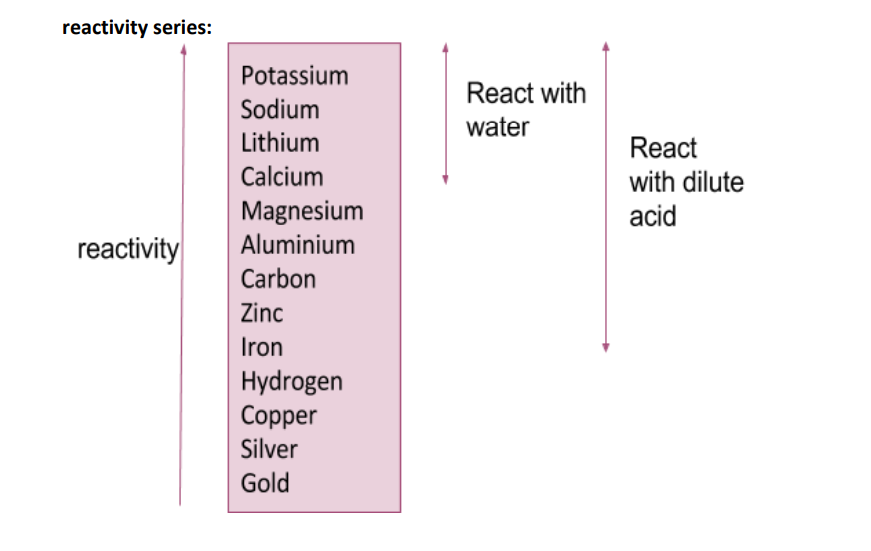
What is the order in the reactivity series of these elements: hydrogen, carbon, calcium, lithium, aluminium, zinc, iron, potassium, magnesium, sodium, gold, silver and copper?
What is rust and what are the conditions under which iron rusts?
Rust is hydrated iron (III) oxide.
Both air and water are necessary for iron to rust – i.e. oxidation – gain of oxygen results in corrosion.
How can the rusting of iron be prevented by: barrier methods, galvanising and sacrificial protection?
Barrier methods - Coat the iron with paint or grease or plastic or something lower on the reactivity series to exclude water and oxygen.
Galvanising - Coating iron with a more reactive metal like zinc. Even if the Zn is partially scratched off the metal will still oxidise before the iron as it is more reactive.
Sacrificial protection - Put a metal that is more reactive than iron on it. The more reactive metal will oxidise first
What is meant by the terms:
• oxidation
• reduction
• redox
• oxidising agent
• reducing agent
in terms of gain or loss of oxygen and loss or gain of electrons?
oxidation: gain of oxygen OR loss of electrons
reduction: loss of oxygen OR gain of electrons
redox: a reaction in which both oxidation and reduction occur
oxidising agent: causes another reactant to be oxidised and is reduced itself
reducing agent: causes another reactant to be reduced and is oxidised itself
How do you investigate reactions between dilute hydrochloric and sulfuric acids and metals (e.g. magnesium, zinc and iron)?
Metals which are above hydrogen in the reactivity series will react with dilute hydrochloric or sulfuric acid to produce a salt and hydrogen.
metal + acid → salt + hydrogen
For example:
magnesium + hydrochloric acid → magnesium chloride + hydrogen
Mg (s) + 2HCl (aq) → MgCl₂ (aq) + H₂ (g)
This is a displacement reaction.
There is a rapid fizzing and a colourless gas is produced. This gas pops with a lighted splint, showing the gas is hydrogen.
The reaction mixture becomes warm as heat is produced (exothermic).
The magnesium disappears to leave a colourless solution of magnesium chloride.
If more reactive metals are used instead of magnesium the reaction will be faster so the fizzing will be more vigorous and more heat will be produced.