Cram Unit 1: Atomic Structures and Properties (copy)
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
Atom
the smallest unit of matter; they are always neutral p+=e-
Nucleus
protons (+) and neutrons (neutral)
Electrons
Negatively charged; surround the nucleus.
Atomic number
The number of protons
Atomic mass
Protons + neutrons
Isotope
Different forms of atoms for the same element with same number of protons and different number of neutrons.
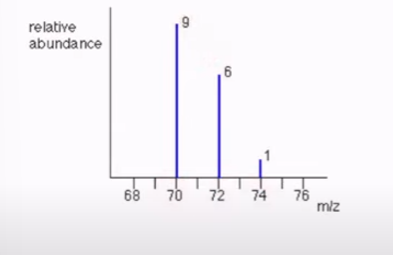
Mass spectroscopy
(% of abundance * mass of the isotope) +(% of abundance * mass of the isotope)…= average atomic mass of an element.
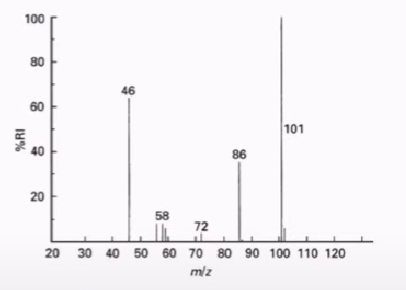
Estimating average atomic mass
You can add the numbers on top to find the percentage of abundance by multiplying (70 × 9/9+6+1)+(72 × 96/9+6+1)… OR find the in-between of the largest columns, here it would be 70 and 71. This means the amu should be around 71.
Classifying Matter
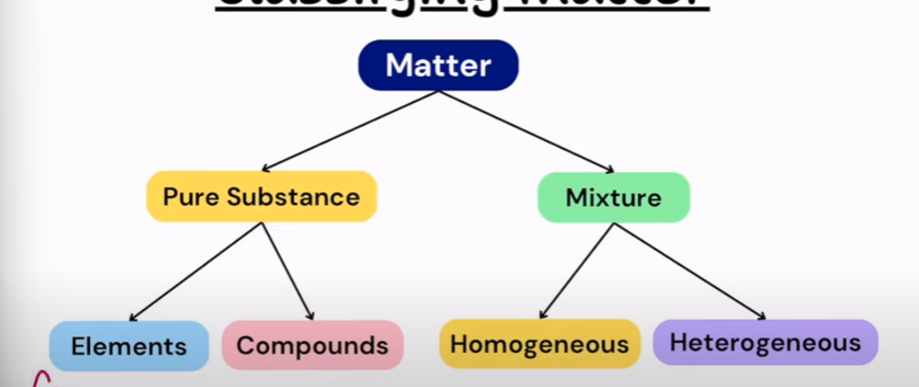
What are the pure substances?
They have invariant chemical composition and have distinct properties. elements and compounds
What are the mixtures?
Two or more pure substances that retain their original identities and can be separated by physical means. Homogeneous and heterogeneous
Element
fundamental substances; cannot be separated into simpler substances. (Cu, Zn, Ag or O2, H2, N2, O3)

Compound
two or more elements in fixed proportions and can be separated into simpler substances/elements by chemical means. (CuO, H2O, CH3CH2OH)
Homogeneous
They have a uniform composition and properties throughout (also called a solution) (Saline water)
Heterogeneous
They are not uniform in composition and properties all throughout. (Salad)
How do we separate mixtures that are completely soluble?
Through evaporation or distillation.
Molar mass (g/mol)
mass of any substance for 1 mole (Atomic mass)
Molar mass for a compound
Just add up the sum of the molar masses respective to the ratio.
Avogrado’s Number
6.022 X 10(23)
Mass
Number of moles * Molar mass
Number of particles
Number of moles * Avogrado’s Number
Number of moles
Mass of the sample / Molar mass
Empirical and Molecular Formula
Molecular C6H12O6 (6:12:6) → Empirical CH2O (1:2:1)
How to calculate molecular formula
Empirical formula + molar mass
Find the molar mass of the empirical formula
Divide the empirical formula molar mass by the molar mass given
Multiply the result to each of the atoms in the empirical formula

Atomic structure
Atom= electrons, protons, and nucleus
Electrons are in specific shells (energy levels) and subshells (sublevels)
Energy shells
n= 1, n=2, n=3, n=4….
Valence electrons
Reside in the outermost shell
Subshells
s, p, d,f
Energy increases from s → f
Shell 1
s
Shell 2
s, p
Shell 3
s, p, d
Shell 4
s, p, d, f
s
2 electrons
p
6 electrons
d
10 electrons
f
14 electrons
s
1 orbital
p
3 orbitals
Hold two electrons each
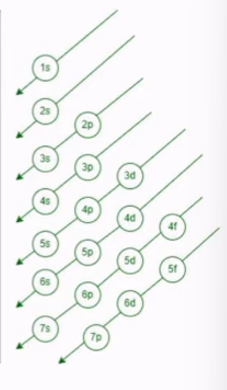
Electron configuration
Aufbau principle: electrons first occupy those orbitals whose energy is the lowest.
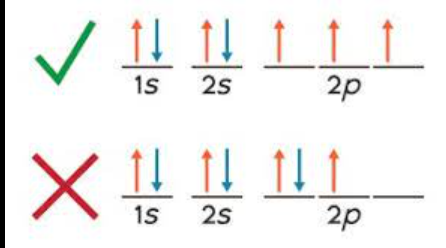
Hund’s Rule
The lowest energy configuration for an atom is the one having the maximum number of unpaired electrons within the same energy sublevel.
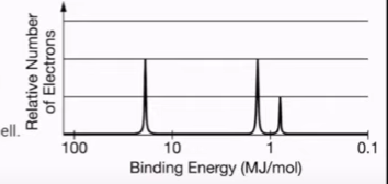
Photoelectron spectroscopy
Represents electron distribution and energy
X-axis: Binding energy (energy necessary to remove an electron)
To the left → Closer to the nucleus
To the right → Closer to the valence shell
Y-axis: # of electrons
Periodic Trends
Atomic radius, ionization energy, electron affinity, electronegativity
Coulomb’s Law
It indicates that forces of attraction depend on the distance and magnitude of the charges.
Greater distance → Less force
Greater magnitude → More force
Atomic radius
As we go down a group, atomic radius increases because there will be more energy levels, maximizing the distance between the nucleus and the valence electrons. Also explained by forces of repulsion as electrons increase, so does the electron shield.
As we go right a period, the atomic radius decreases because the number of protons also increases, creating greater magnitude, thus creating greater forces of attraction.
Ionic radius
If they lose electrons, they become smaller.
If they gain electrons, they become larger.
First ionization energy
The amount of energy required to remove an electron.
Ionization energy trend
As electrons are closer to the nucleus, energy increases because of the high nuclear charge.
As electrons are farther away from the nucleus, energy decreases because of the weaker nuclear charge.
It is likely that ionization energy will be lower if the subshell is not…
fully-filled.
Electron affinity
The energy change that occurs when an atom gains an electron.
Electronegativity
The ability of an atom to attract electrons.
Increases from left to right because effective nuclear charge increases
Decreases down a group because there are more shells, creating less attractions between the nucleus and electrons.