Biochem Session 9- Carbohydrates/Fatty Acids and Lipids
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
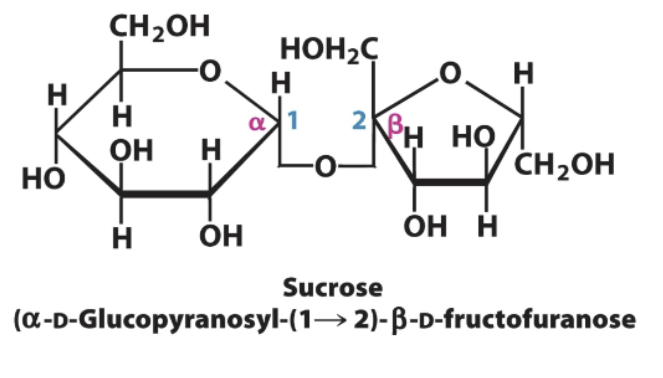
Sucrose→ monosaccharides and bond type
Glucose→Fructose
Linkage is alpha for glucose and beta for fructose (1→2)
Glucose in alpha, fructose in beta
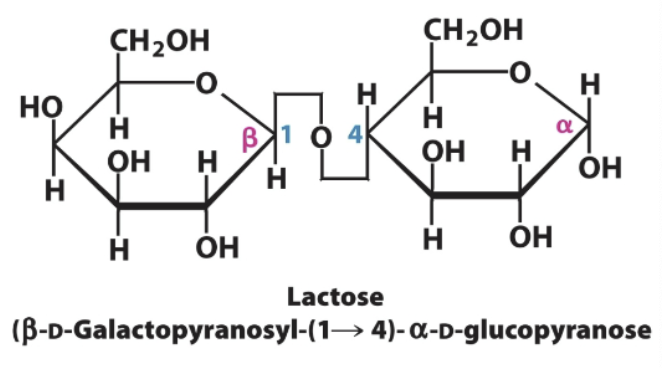
Lactose→ monosaccharides and bond type
Galactose→Glucose
B-1,4-glycosidic bond
Galactose in beta, glucose in alpha
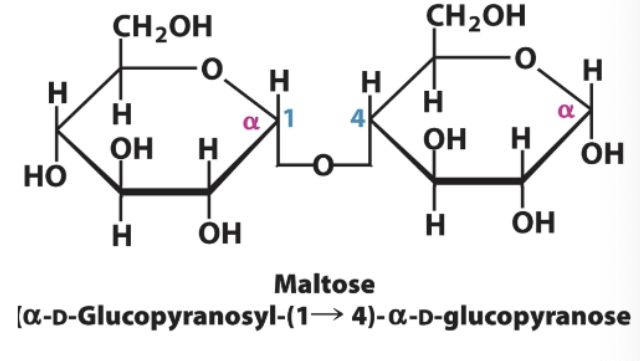
Maltose→ monosaccharides and bond type
Glucose→Glucose
a-1,4-glycosidic bond
Glucose 1 in alpha, glucose 2 in alpha
Define “aldose” and “ketose.”
Aldose→ sugar containing an aldehyde moiety (carbonyl at C1)
Ketose→ sugar containing a ketone moiety (carbonyl at C2)
What is the “anomeric carbon” in a monosaccharide, and why is it important?
The anomeric carbon is linked to both the aldehyde or ketone moiety in the open chain form and the anomeric hydroxyl that can form glycosidic bonds/position determines anomeric isomer designation (a/B) in the cyclic form
What is mutarotation, and which carbon atom’s stereochemistry is changing during this process?
Mutarotation is the active fluctuation between a/B anomers→ position of the -OH is changing about the anomeric carbon
Compare α- and β-glycosidic linkages and give an example of a disaccharide with each.
α-glycosidic linkages→ located above the plane of the ring
Ex: Maltose→ α-1,4 linkage between two glucoses
β-glycosidic linkages→ located above the plane of the ring
Ex: Lactose→ β-1,4 linkage between galactose and glucose
Name the three most common disaccharides and list their component monosaccharides.
Sucrose→ Glucose (alpha) and fructose (beta) 1→2
Lactose→ Galactose (beta) and glucose (alpha) B-1,4
Maltose→ Glucose (alpha) and glucose (alpha) a-1,4
Describe the main structural difference between amylose, amylopectin, and glycogen.
Amylose→ linear/all a-1,4 linkages
Amylopectin→ branched/1 a-1,6 linkage for every 30 a-1,4 linkages
Glycogen→ most branched/1 a-1,6 linkage for every 8 a-1,4 linkages
What are glycoproteins? Give two examples of where they are commonly found.
Glycoproteins→ proteins covalently bonded to a carbohydrate (protein=major component and carbohydrate=minor component)
Commonly found in biological membranes (receptors) and extracellular matrix proteins
What is the repeating disaccharide unit of chitin?
β-1,4 linked N-acetyl-D-glucosamine
Define “essential fatty acids” and give one example
Essential fatty acids are fatty acids that are required in the diet but are not produced by humans
Ex: linoleic acid, α-linolenic acid
What is the difference between cis and trans double bonds in fatty acids, and what health impacts are associated with trans fats?
Cis double bonds→ cause kinks in the acyl chain (alkene H’s on same side)
Trans double bonds→ allow acyl chain to be more linear (alkene H’s on opposite sides)
Trans fats industrially produced and are associated with coronary heart disease, inflammation, etc.→ form stronger VdW interactions and human’s cannot effectively break them down
What is meant by “omega-3 fatty acid”? Give one example.
First double bond in a saturated fatty acid is located 3 carbons from the omega (terminal/methyl) carbon.
Ex: EPA (20:5 Δ5,8,11,14,17) is an omega-3 FA
Name the three major classes of membrane lipids
Phospholipids
Sphingolipids
Cholesterol
What is the structural difference between glycerophospholipids and sphingolipids?
Glycerophospholipids→ glycerol backbone
Sphingolipids→ sphingosine backbone
What is a liposome, and how are they used in biomedical research?
A liposome is a lipid vesicle made of a phospholipid bilayer→ has an inner aqueous compartment lined with H-philic heads and outer aqueous compartment (interacts with solution) lined with H-philic heads. H-phobic tails interact with each other within the bilayer
They are used for drug delivery (drug placed in inner aqueous compartment) and for studying membrane proteins
What is the driving force for spontaneous bilayer formation?
Hydrophobic interactions/the hydrophobic effect→ nonpolar tails cluster away from water and interact with each other to form the bilayer
Which molecule makes up roughly 25% of plasma membrane lipids in some nerve cells but is absent from some intracellular membranes?
Cholesterol
In what way are cerebrosides and gangliosides structurally different?
Both contain a sphingosine backbone
Cerebrosides→ contain 1 sugar group (glucose or galactose)
Gangliosides→ contain up to 7 sugar groups
Define “micelle” and explain why fatty acids form them in aqueous solution.
A micelle is a spherical aggregate of fatty acids that forms in aqueous solution as a result of the hydrophobic effect (clustering of nonpolar tails). Fatty acids are cylindrical in shape (single tail→ head cross section larger than tail cross section), so they favor the formation of spherical aggregates
Draw the chair and boat conformations of glucose. Which is energetically favored and why?
Chair is more stable because all of the axial groups are H’s and all bulky groups are in the equatorial position (more stable due to reduced steric clash)
You dissolve pure α-D-glucose in water and measure its optical rotation over time. What do you expect to observe and why?
The optical rotation would continue changing until equilibrium between anomers is established
You test sucrose and maltose with Fehling’s reagent. Which one gives a positive test and why?
Maltose because it has a free anomeric -OH that can reduce Cu2+ to Cu+ which will form a red precipitate with water→ positive. Sucrose does not have a free anomeric carbon and is non-reducing→ negative.
Explain how glycogen’s branching pattern contributes to its rapid mobilization compared to amylose.
Glycogens branching pattern allows it to have more glycosidic bonds than amylose. This means it can undergo rapid glycogen phosphorylase activity→ releases glucose more efficiently to meet metabolic needs
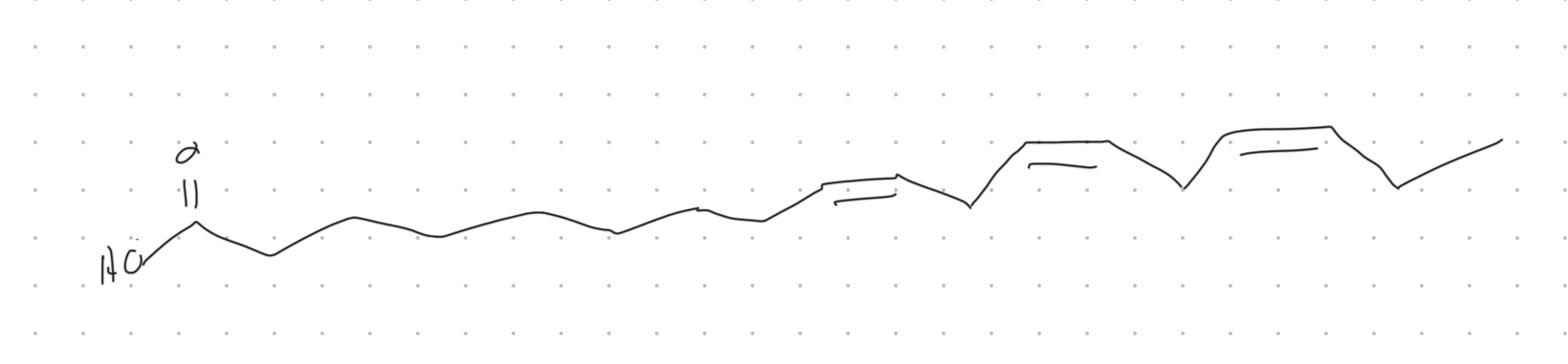
Given a fatty acid notation “18:3 all cis-Δ9, Δ12, Δ15,” draw its general structure and classify it as omega-3 or omega-6
Omega-3 polyunsaturated fatty acid
If a cell membrane had a very high cholesterol content, how would this affect membrane fluidity at (a) low temperature and (b) high temperature?
Low temp→ increased fluidity
The bulky nature of cholesterol’s polycyclic ring structure interrupts VdW interactions between fatty acid chains (prevents packing) that stabilize the gel phase
High temp→ decreased fluidity
The constrained nature of cholesterol’s polycyclic ring structure restricts movement (lateral diffusion) in the membrane
Explain how partial hydrogenation of vegetable oil leads to trans fats, and why this is a health concern.
Partial hydrogenation converts some cis double bonds to trans→ results in straighter chains that are more difficult to break down→ leads to inflammation and coronary heart disease