7.1: chemical equilibria: reversible reactions, dynamic equilibrium
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
Define a reversible reaction
A reaction in which products can be turned back into reactants by reversing conditions
In dynamic equilibrium:
The rate of the forwards and backwards reaction is the same in a closed system
The concentrations of the reactants and products are constant
When does the concentration stop changing in a reaction?
When equilibrium is reached
What does Le Chatelier’s principle state?
if a change is made to a system at dynamic equilibrium, the position of the equilibrium moves to minimise this change
If the concentration of a reactant is increased:
Equilibrium shifts to the right
If the concentration of a product is increased
Equilibrium shifts to left
If pressure is increased:
Equilibrium shifts in the direction that produces a smaller number of molecules of gas to decrease the pressure again
If temperature is increased
Equilibrium moves in the endothermic direction to reverse the change
If temperature is decreased
Equilibrium moves in the exothermic direction to oppose the change
Explain the effect of catalysts
Substance that increases the rate of a chemical reaction
Increase the rate of the forward and reverse reaction equally
Only cause a reaction to reach its equilibrium faster
Catalysts therefore have no effect on the position of the equilibrium once this is reached
What is Kc defined as
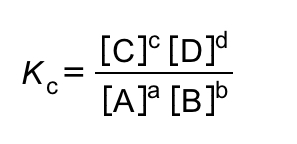
Which state is ignored in Kc expressions?
Solids
Define partial pressure
The pressure exerted by particular gas A in a mixture of gases
Mole fraction=
The number of moles of a particular gas/ total number of moles of all gases in a mixture
Partial pressure =
Mole fraction x total pressure
What is the only factor that can change Kc?
Temperature
Explain how ammonia yield is maximised in the haber process in terms of pressure
Increasing pressure shifts equilibrium to the right, increasing ammonia yield
Higher pressure also increases collision frequency, enhancing the reaction rate
However, very high pressures are costly and require strong containment
Compromise pressure used
≈ 200 atm
Explain how ammonia yield is maximised in the haber process in terms of temperature
The forward reaction is exothermic
Lowering temperature shifts equilibrium to the right, favouring ammonia formation
But too low a temperature would slow the reaction rate, delaying equilibrium
Compromise temperature:
400–450 °C
Explain how ammonia yield is maximised in the haber process in terms of removing ammonia
Ammonia is removed by cooling and condensing it to a liquid
This shifts the equilibrium further to the right, producing more ammonia
Stored ammonia is kept at low temperatures where decomposition is very slow, especially in the absence of a catalyst
Explain how ammonia yield is maximised in the haber process in terms of using a catalyst
An iron catalyst is used to increase the rate of reaction without affecting equilibrium position
Without it, the reaction would be too slow
Explain how ammonia yield is maximised in the contact process in terms of pressure
Increasing pressure shifts equilibrium to the right, favouring SO3 formation
However, the equilibrium constant (Kp) is already very large at low pressures
Industrial pressure used:
~1 atm
Explain how ammonia yield is maximised in the contact process in terms of temperature
Reaction is exothermic
Lower temperatures would favour SO3 production, but also reduce the rate
Compromise temperature:
≈ 450 °C
Explain how ammonia yield is maximised in the contact process in terms of removing sulfuric acid
Shifts equilibrium to the right, driving reaction forward
Explain how ammonia yield is maximised in the contact process in terms of using a catalyst
Contact process uses vanadium(V) oxide as a catalyst to increase the rate of reaction