BILD 1 - Midterm #2
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
56 Terms
Recognize that under biological conditions, matter and energy are each conserved.
Matter and energy each have their own laws of conservation - they don't actively turn into each other. Therefore, mass must become another form of mass and energy must become another form of energy.
Distinguish between a spontaneous and a non-spontaneous reaction.
A spontaneous reaction has reactants with a higher amount of energy than the products, and as such the reaction occurs naturally, as the amount of energy decreases overall. A non-spontaneous reaction has products that have a higher amount of energy than the reactants, so the reaction does not occur naturally as it needs energy to prompt the increase of energy from the react to the product stage.
Use ΔG to distinguish between exergonic and endergonic reactions.
ΔG refers to Gibbs free energy, where positive refers to an increase and negative refers to a decrease. Spontaneous (same as exergonic) releases energy, so it corresponds with negative ΔG. Non-spontaneous (same as endergonic) absorbs energy, so it corresponds with a positive ΔG.
Explain how exergonic reactions, such as hydrolysis of ATP, are paired with endergonic reactions to drive them in cells.
Endergonic reactions cannot occur on their own, as otherwise they can never gain the energy to form the higher-energy products. Therefore, the energy released by an exergonic reaction is paired with an endergonic reaction in order to cause the endergonic reaction to progress.
Predict and explain how the presence of an appropriate enzyme will affect the rate of a reaction inside the cell.
A catalyst lowers the activation energy needed to prompt a reaction. This means that with less energy needed to prompt the reaction, the reaction will generally occur faster in the cell.
Use conservation of energy and mass, the willow experiment, and the oxygen labeling experiment to explain the source of new biomass in plants.
Energy in plants comes directly from sunlight, which is absorbed by chlorophyll in the chloroplasts. The source of mass in a tree (and other plants), based on the willow experiment, is CO2 from the environment.
Trace the origin of the specific atoms and energy that are in the products of photosynthesis.
The oxygen labeling experiment shows that the atoms in CO2 gas become a part of glucose (which the plant uses to grow), while the oxygen atoms from water become a part of O2, which is released. The hydrogen atoms, on the other hand, form glucose. The energy from plants comes directly from sunlight, which is what allows energy to be stored in glucose's bonds.
Explain and distinguish between the light reactions and the Calvin cycle in photosynthesis in terms of roles, inputs, and outputs.
Light Reactions: Takes in sunlight and water, produces ATP and NADH for the Calvin Cycle, and makes O2 as a byproduct.
Calvin Cycle: Takes in CO2, uses ATP and NADH (making ADP and NAD+) for the light reactions, and makes 2 molecules of G3P (glyceraldehyde 3-phosphate), which eventually become glucose.
Describe the inputs, processes, and products of aerobic cellular respiration as a whole and of glycolysis, transition reaction, citric acid cycle, and oxidative phosphorylation.
Overall Process Equation: C6H12O6 + 6O2 ---> 6CO2 + 6H2O + (36-38) ATP (this comes from ADP and phosphate groups that come in together)
Glycolysis: In the cytoplasm, takes glucose apart into 2 pyruvate molecules, producing 2 NADH and 2 ATP.
Transition Reaction: In the mitochondrial matrix, 2 pyruvate molecules form 2 CO2 molecules and create 2 NADHs, then form 2 Acetyl CoA with 2 Coenzyme A molecules.
Citric Acid Cycle: 2 Acetyl CoA goes through an 8-step cycle resulting in a smaller sugar (oxaloacetate) and the return of CoA. More importantly, however, it forms 4 CO2 , 6 NADH, 2 FADH2 , and 2 ATP (half for each molecule).
Oxidative Phosphorylation: All 10 NADH and 2 FADH2 use their energy to push their H+ ions into the intermembrane space (using four complexes). Then, H+ comes back through the pump into the inner membrane forming 32-34 ATP and with O2, creating H2O.
Predict how perturbations of the inputs and processes of the stages of aerobic cellular respiration (i.e. glycolysis, transition reaction, citric acid cycle, and oxidative phosphorylation) would affect the other processes of cellular respiration and the number of ATP made.
If any one stage is affected, subsequent stages would also likely suffer. The worst thing to happen would be the absence of glucose, because the cell couldn't gain any energy at all. However, if there's glucose but no oxygen, then just glycolysis and fermentation occurs (this only produces 2 ATP). If any of the subsequent stages are interrupted in any meaningful way, then potential reactions are: excess NADH/FADH2, excess O2 not being used, low levels of CO2 respiration, excess of any one stage's intermediate products (such as pyruvate or acetyl CoA), and damage to the mitochondria.
Trace the origin of the specific atoms and energy that are in the products of cellular respiration.
Energy in cellular respiration comes from sunlight, then plant-produced glucose, until it eventually ends up in ATP as produced by cellular respiration. The carbon comes from CO2, which joined with H2O to form glucose, and now forms CO2 again in cellular respiration. Similarly, the H in glucose comes from water initially and returns to water after cellular respiration. The O in CO2 produced by cellular respiration originally came from CO2 as well, and the O in H2O originally came from H2O.
Trace how carbon atoms and energy transfer from organism to organism in an ecosystem.
When an organism (usually a consumer) dies, it is decomposed by a decomposer, which releases CO2. Then, the CO2 is absorbed by a plant and used to form glucose. Then, a consumer eats a plant and uses the glucose to make CO2. Energy in plants comes from the sunlight, which is then stored in glucose. A consumer and then eventually a decomposer can take that glucose and gain that energy.
Use the concepts of photosynthesis and cellular respiration to predict the outcome of experiments on the relative biomass of different organisms.
Photosynthesis needs light, water, and carbon dioxide concurrently in order to occur, but cellular respiration can occur anaerobically with glucose already pre-stored, which means that organisms can lose mass overall by using up their stores of glucose and continuing to perform cellular respiration. (See the radish experiment.)
Misconception: A positive ΔG means that a reaction will occur spontaneously.
A negative ΔG means that a reaction will occur spontaneously.Remember that in a spontaneous reaction, products have less energy than reactants; a small number - a big number = < 0.
Misconception: Spontaneous reactions (those with a negative ΔG) always happen frequently and quickly.
Spontaneous reactions may occur frequently and quickly. However, it depends on the activation energy of the reaction. If the activation energy is very high, the reaction may not proceed very quickly or frequently in real life.
Misconception: Exergonic and endergonic mean the same thing.
They are opposites! Exergonic refers to a reaction with a negative ΔG, and exergonic reactions are spontaneous. Endergonic refers to a reaction with a positive ΔG, and endergonic reactions are nonspontaneous.
Misconception: Exergonic / endergonic reactions are the same as exothermic / endothermic reactions.
Exothermic and endothermic are about whether a reaction releases heat, while exergonic and endergonic are about whether a reaction releases energy. Heat is one type of energy, but not the only one.
Misconception: An exergonic reaction will always release heat.
An exergonic reaction may release heat energy, but it may also absorb heat energy if other types of energy are released.
Misconception: Endergonic reactions (those with a positive ΔG) can never happen.
Endergonic reactions can happen if they are coupled with an exergonic reaction and the ΔG of the sum of the two reactions is negative. The cell often uses the following reaction as the exergonic reaction: ATP→ ADP + a phosphate ion (Pi).
Misconception: Enzymes can make non-spontaneous reactions spontaneous.
Enzymes do not change the ΔG of a reaction (or whether it is spontaneous or not). They lower the activation energy.
Misconception: Enzyme shape does not matter.
The shape of an enzyme determines what it will bind to and what chemistry it can help its reactants do.
Misconception: An enzyme is a reactant.
Enzymes are not reactants or products. They are catalysts that are not used up or made during the reaction.
Misconception: Enzymes are used up during chemical reactions.
Enzymes are not used up and can be used again and again. They may participate in the reaction chemically, but another chemical reaction always happens to restore them to their original state.
Misconception: Enzymes work equally well in all conditions.
Enzymes that evolved in certain conditions, like at a particular acidity or temperature, usually work best in those conditions. The further the conditions are away from those conditions, the less well the enzyme will work.
Misconception: The dry mass of a plant comes mostly from the soil.
Soil contributes trace elements to the plant like some nitrogen, phosphorus, and metal ions, and the plant also absorbs water from the soil. But most of the dry mass of a plant comes from the carbon, which is not from the soil. (See the willow experiment.)
Misconception: The dry mass of a plant comes mostly from water.
A living plant has a lot of water in it, but when we measure dry mass, we dry the plant out and do not count this water. Also, the oxygen atoms in water do not contribute to the glucose a plant makes but rather turn into oxygen gas in the light reactions. (See the oxygen labeling experiment and the light reactions.)
Misconception: The dry mass of a plant comes mostly from sunlight.
Sunlight contributes energy. Energy is not converted to mass in chemical reactions. Therefore, sunlight energy does not contribute mass to the plant.
Misconception: Air has no mass.
Air has many molecules in it like carbon dioxide, and these have mass.
Misconception: The light reactions and Calvin cycle do the same thing.
The light reactions harvest sunlight energy to form carrier molecules like ATP and NADPH. The Calvin cycle uses those energy carriers to turn carbon dioxide into glucose.
Misconception: The Calvin cycle only occurs in the dark.
The Calvin cycle can occur in either the light or the dark. It just doesn't directly require light.
Misconception: The Calvin cycle never requires light.
The Calvin cycle doesn't directly require light, but it needs the products of the light reactions. So, if you leave a plant in the dark for a long time, the plant will use up its ATP and NADPH and stop being able to do the Calvin cycle too.
Misconception: When an organism is dead, its carbon atoms return to the ecosystem through
direct breakdown of its molecules.
When an organism is dead, its carbon atoms return to the ecosystem through decomposers like bacteria and fungi eating their molecules and respiring the carbon out as carbon dioxide. Purely chemical breakdown would be too slow.
Misconception: Plants absorb organic molecules from dead organisms through their roots in the soil.
Plants get their carbon from carbon dioxide in the air. They largely do not absorb organic molecules through their roots.
Misconception: When an organism is dead, its own reactions convert carbon atoms into carbon dioxide that is released to the atmosphere.
Dead organisms do not do cellular respiration or many chemical reactions at all. They decompose because decomposers consume them.
Misconception: In cellular respiration, the oxygen in CO2 comes from oxygen gas.
In cellular respiration, the oxygen in the CO2 comes from the glucose.
Misconception: Oxygen gas that is breathed in becomes part of carbon dioxide.
As part of cellular respiration, the oxygens in oxygen gas are turned into water.
Misconception: All the stages of cellular respiration directly require O2 to function.
Only the electron transport chain directly requires O2. However, if the electron transport chain is not working, the citric acid cycle will run out of NAD+ and FAD, so it will stop too.
Misconception: If one stage of cellular respiration is blocked, subsequent ones will be able to function.
If one stage of cellular respiration is blocked, the ones after it will not have their inputs, so they will not be able to function either.
Misconception: If levels of a certain molecule are high, that must be an issue with the stage of respiration that produced them.
If levels of a certain molecule are high, there might be an issue with the stage of respiration that produced them. However, there might instead be an issue with the next stage of respiration, which is not using up that molecule and therefore causing levels to be high.
Misconception: Organisms cannot lose mass by respiration.
When organisms lose mass, the main way they lose carbon is through cellular respiration. This is a major way they lose oxygen too, as the oxygen in CO2 also comes from the organic molecules being broken down in cellular respiration. Each individual CO2 doesn't weigh a lot, but breath by breath it adds up.
Misconception: Plants do not do cellular respiration.
Plants do cellular respiration. Once they create sugars using photosynthesis, they must break them down to get ATP for their own energy needs.
Misconception: Plants only do cellular respiration at night.
Plants do cellular respiration all the time to create ATP for their energy needs.
Misconception: Plants only have chloroplasts, not mitochondria.
Plants have both chloroplasts and mitochondria, as they have mitochondria to do cellular respiration to create ATP.
Misconception: Plants do not consume O2 or release CO2.
Plants do cellular respiration, and as a result they consume O2 and release CO2. However, they also release O2 and consume CO2 as part of photosynthesis. A healthy plant will tend to release more O2 than it consumes and consume more CO2 than it releases.
When does the primary structure of a molecule change?
Primary structure depends on the order of amino acids - if the order/make-up of the amino acid chain changes, then so too does the structure.
When does the secondary structure of a molecule change?
Secondary structure refers to types of structure, such as an α helix and a β pleated sheet, determined by interactions between molecules in the backbone. As such, when the backbone is interacted with, then the secondary structure will change.
When does the tertiary structure of a molecule change?
Tertiary structure depends on interactions between R groups in amino acids, such as disulfide bridges, hydrogen and ionic bonding, and dispersion forces. As such, if amino acids in the chain change, then the tertiary structure will change.
When does the quaternary structure of a molecule change?
Quaternary structure depends on interactions between R groups in amino acids between separate larger protein molecules. If amino acids in a chain change, then different protein molecules may be attracted or repelled unlike before.
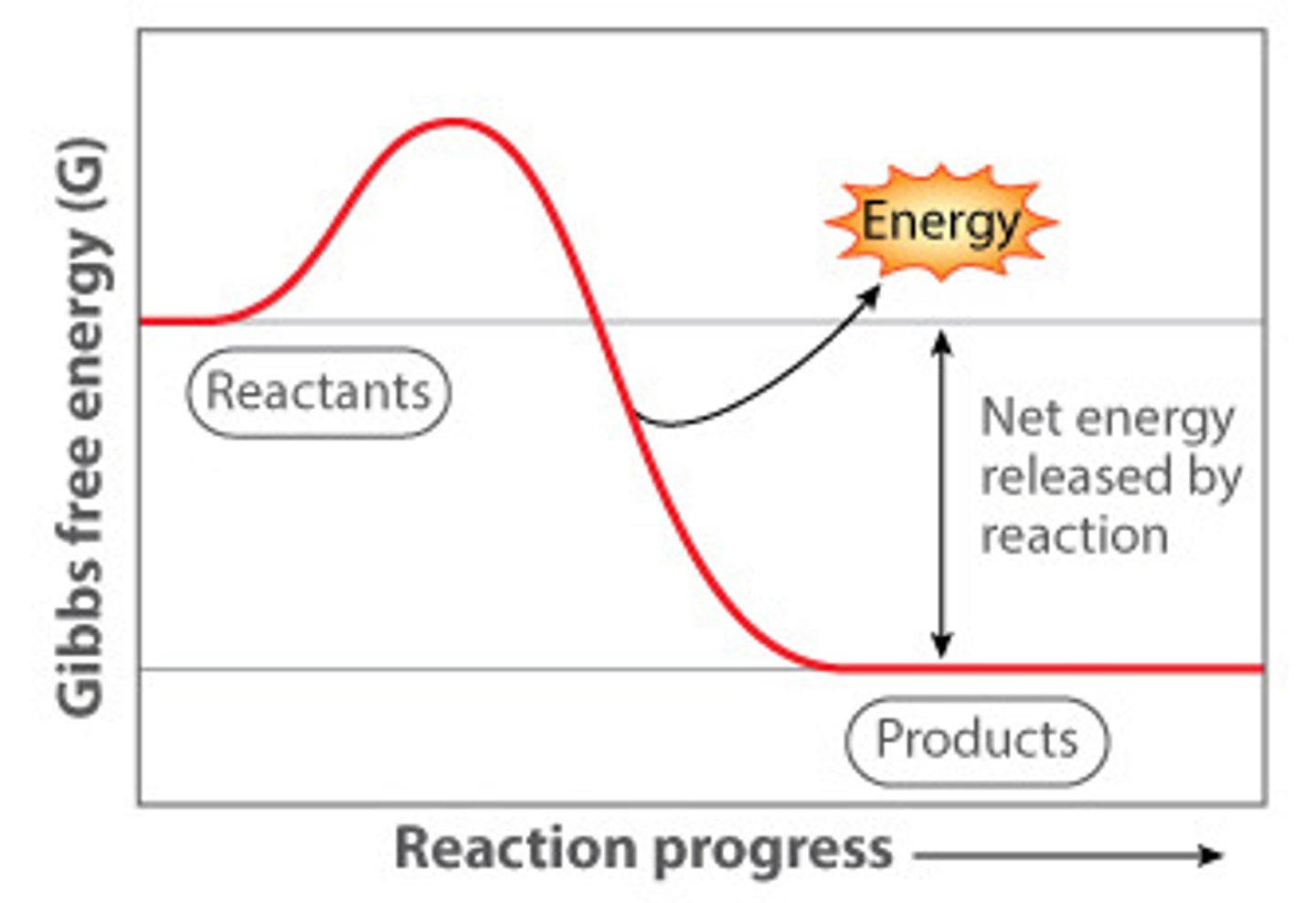
What does an energy diagram for an exergonic reaction look like?
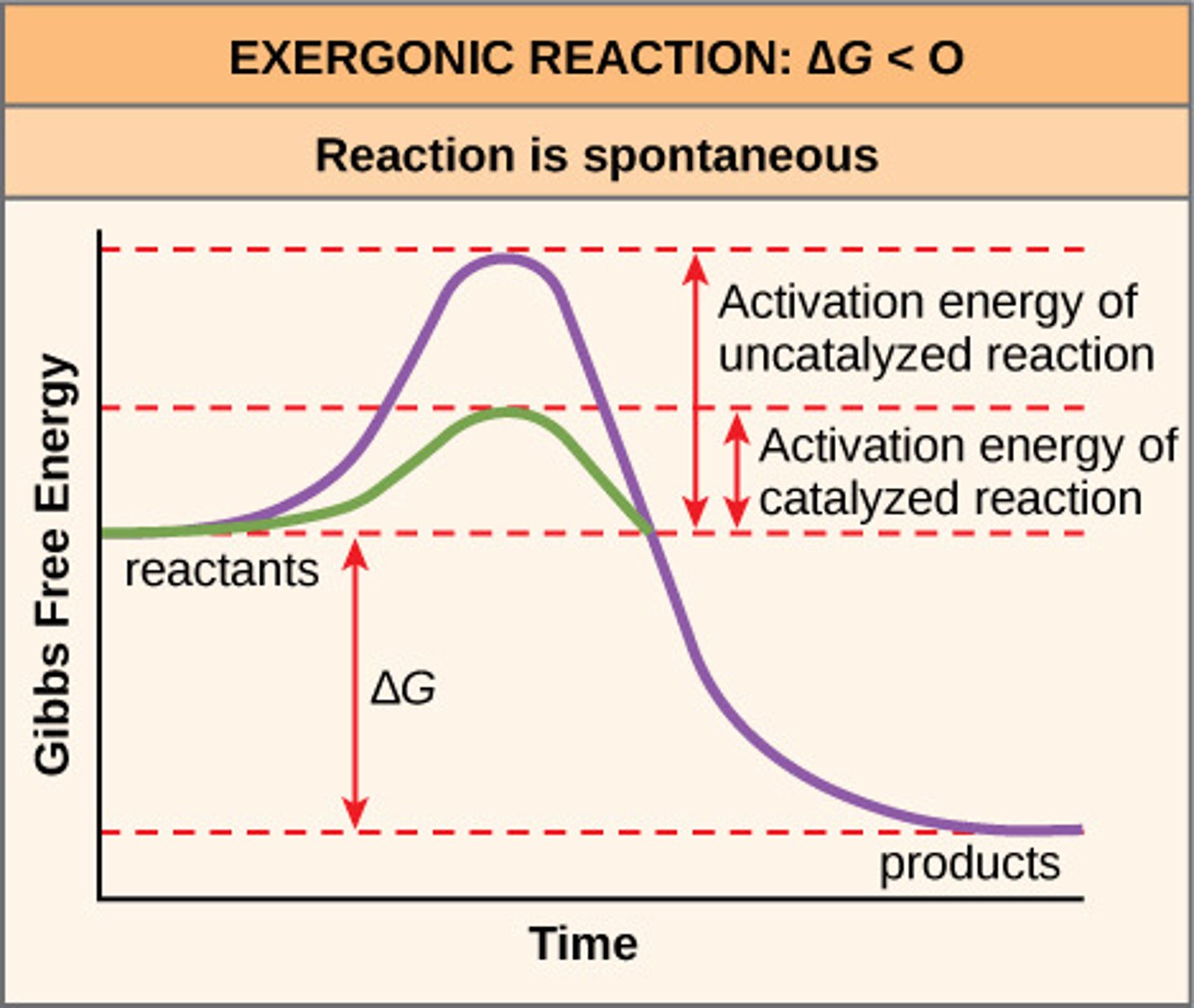
What does an energy diagram for an exergonic reaction look like with an enzyme?
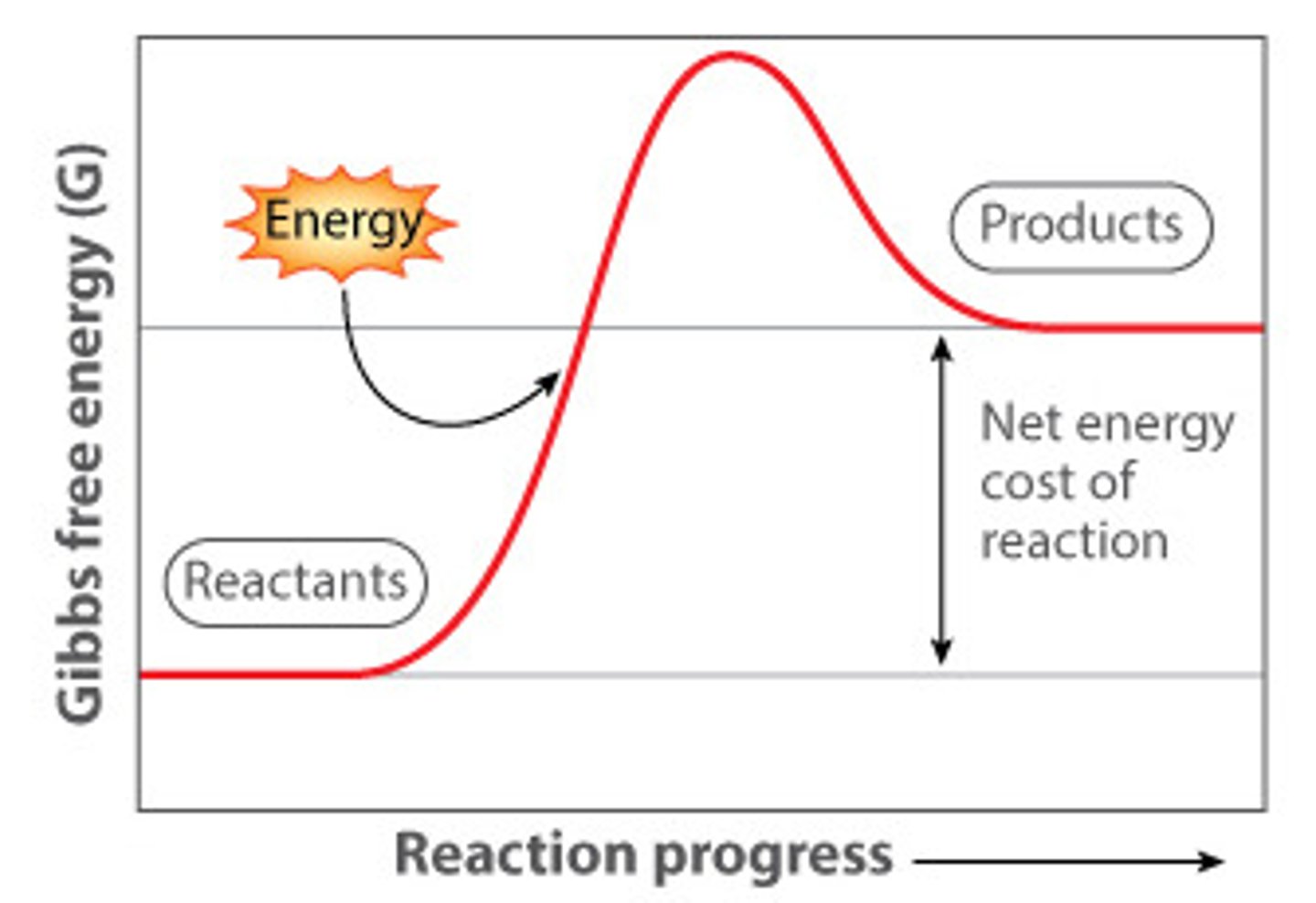
What does an energy diagram for an endergonic reaction look like?
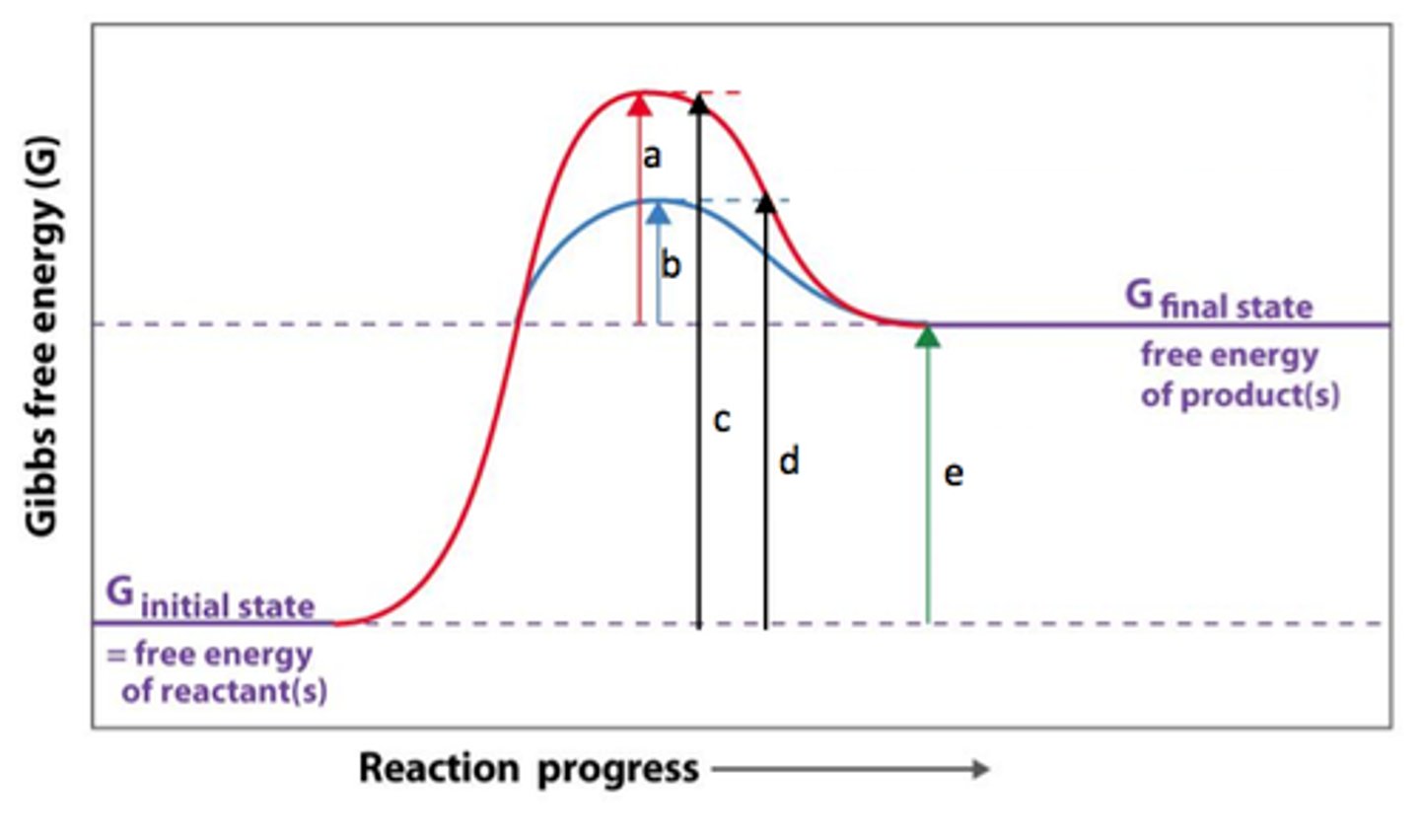
What does an energy diagram for an endergonic reaction look like with an enzyme?
Predict how changes in the environment of an enzyme that has evolved in a particular context might the rate of the reaction it catalyzes.
Enzymes are usually proteins (and occasionally RNA molecules) that only work in their preferred environments, based primarily on temperature and acidity. If a protein has their environment changed, then they will denature and change their shape. Because the function of enzymes depends on their shape, the enzyme is no longer able to do its job effectively and so the rate of reaction will not increase.
Predict how changes to the shape of a potential substrate or the side chains at the active site of an enzyme might affect the rate of catalysis.
An enzyme functions by binding to the substrate, which is based on how their shapes fit together. If the substrate's shape or the side chains in the enzyme change, then the enzyme will not fit with the substrate properly, and as such, the catalysis won't really be effective.
What is one way a virus can enter a cell if it is too big to go through with a channel/pump?
Endocytosis or phagocytosis, which transports large or a lot of substance at once by having the cell temporarily change shape to engulf the substances.
If two molecules with hydrophilic side chains turn into two molecules with hydrophobic side chains, how will they interact differently with each other?
If they are in water, then the molecules with the hydrophilic side chains will repel each other in the water and the ones with the hydrophobic side chains will attract each other while repelling the water.