Atomic Structure, Bonding, and Periodic Table Concepts in Chemistry
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
68 Terms
Average Atomic Mass
This is the weighted average of the atomic masses of the isotopes in the naturally occurring element, expressed in atomic mass units (u).
Pure Covalent Bond
A covalent bond in which electrons are shared equally between the two atoms because they have the same or very similar electronegativities.
Iron(II)
Ferrous, represented as Fe²⁺.
Iron(III)
Ferric, represented as Fe³⁺.
Copper(I)
Cuprous, represented as Cu⁺.
Copper(II)
Cupric, represented as Cu²⁺.
Tin(II)
Stannous, represented as Sn²⁺.
Tin(IV)
Stannic, represented as Sn⁴⁺.
Lead(II)
Plumbous, represented as Pb²⁺.
Lead(IV)
Plumbic, represented as Pb⁴⁺.
Antimony(III)
Stibnous, represented as Sb³⁺.
Antimony(V)
Stibnic, represented as Sb⁵⁺.
Cobalt(II)
Cobaltous, represented as Co²⁺.
Cobalt(III)
Cobaltic, represented as Co³⁺.
Gold(I)
Aurous, represented as Au⁺.
Gold(II)
Auric, represented as Au²⁺.
Mercury(I)
Mercurous, represented as Hg⁺.
Mercury(II)
Mercuric, represented as Hg²⁺.
Dmitri Mendeleev
Father/Master of the periodic table who arranged elements in horizontal rows in order of increasing atomic mass.
Mendeleev's Periodic Law
When elements are arranged in order of increasing atomic mass, their properties show a periodic recurrence and gradual change.
Modern Periodic Law
When elements are arranged in order of increasing atomic number, their properties show a periodic recurrence and gradual change.
Cathode Ray Tube experiment
An experiment where a beam originated from the cathode and was bent in magnetic/electric fields, indicating the presence of charged particles.
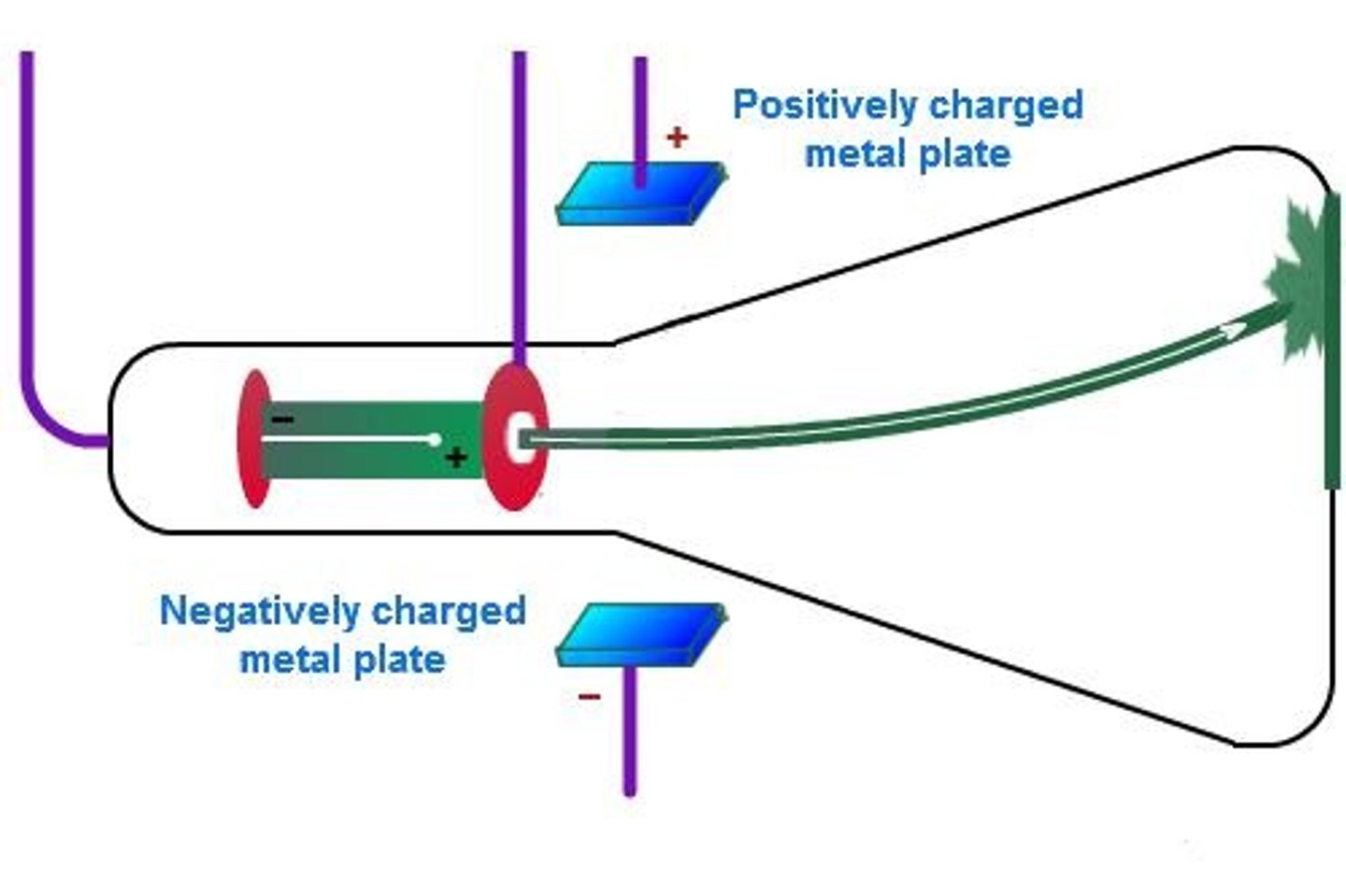
Electrons
Negatively charged particles in atoms.
Plum Pudding Model
A model of the atom where positively charged spheres have electrons scattered throughout.
Gold Foil Experiment
An experiment where most alpha particles went through gold foil, but some were deflected, indicating something dense inside the atom.
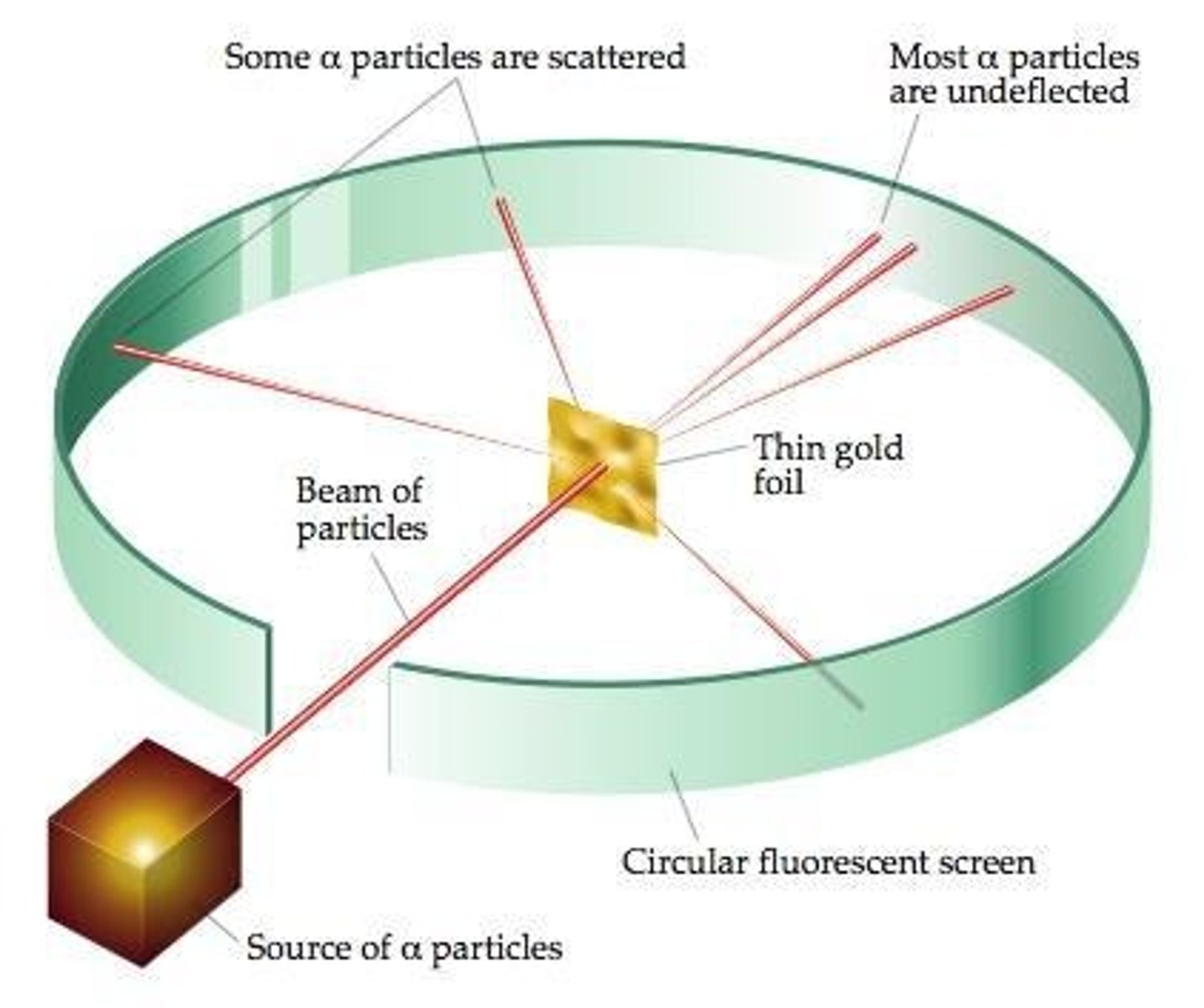
Atoms
Mostly empty space with a small, dense, positive nucleus.
Nuclear Model
A model where electrons orbit a central nucleus randomly at great distances.
Quantized orbits
Specific energy levels that electrons occupy.
Energy Level (n)
A specific level where electrons can exist, with a maximum capacity defined by 2n².
Max Capacity (2n²)
The maximum number of electrons that can occupy a given energy level.
Planetary Model
A model where electrons orbit the nucleus like planets around the sun.
Neutron
A neutral particle in the nucleus discovered by James Chadwick.
Modern periodic Table
A table with 7 periods along the horizontal axis and 18 groups/families along the vertical axis.
Alkali metals
Elements found in group one of the periodic table.
Alkaline earth metals
Elements found in group two of the periodic table.
Transition metals
Elements found in groups 3-12 of the periodic table.
Noble gases
Elements found in group 18 of the periodic table.
Atomic Radius
One half the distance between the nuclei of two atoms of the same element, indicating atomic size.
Ionization Energy
The amount of energy required to remove an electron from an atom or ion in the gaseous state.
Electronegativity
A number that describes the relative ability of an atom to attract electrons when bonded.
Electron Affinity
A measure of the attraction between an incoming electron and the nucleus.
Bond polarity
Describes the distribution of electrical charge around a bond, determined by the difference in electronegativity (ΔEN).
Bond dipole arrows
Arrows that point towards the atom with higher electronegativity in a bond.
Molecular Compounds
Compounds that tend to be solids, liquids, or gases at SATP and do not conduct electricity in solution.
Shared pairs
Electron pairs drawn between element symbols, representing bonding pairs.
Lone pairs
Electron pairs drawn next to only one symbol, representing non-bonding pairs.
H2O
water
NH3
ammonia
C6H12O6
glucose
C12H22O11
sucrose
CH4
methane
C3H8
propane
C2H5OH
ethanol
Diatomic Molecules
H2 (g), N2 (g), O2 (g), F2 (g), Cl2 (g), Br2 (l), I2 (s)
Binary Acids
Acid composed of hydrogen + non-metal
IUPAC Naming (Gaseous & Aqueous Forms)
Name the compound as aqueous hydrogen [non-metal stem]. No prefixes are used, even though it's a molecular compound. Only used when specifying physical state (e.g., (g), (aq)).
Classical Naming
"Hydro" + stem of non-metal + "ic acid". Only used for aqueous solutions of hydrogen + non-metal compounds.
H₂S (g)
hydrogen sulphide
H₂S (aq)
aqueous hydrogen sulphide
Binary acids must be aqueous (aq)
to be named using the classical system.
Oxyacids
Acid composed of hydrogen + polyatomic ion (contains oxygen)
IUPAC Naming for Oxyacids
Name as aqueous hydrogen [polyatomic ion name]. E.g., aqueous hydrogen sulphate.
Classical Naming Rules for Oxyacids
"ic acid" → if the polyatomic ion ends in -ate; "ous acid" → if the polyatomic ion ends in -ite.
H₂SO₄ (g)
hydrogen sulphate
H₂SO₄ (aq)
aqueous hydrogen sulphate
HIO (g)
hydrogen hypoiodite
HIO (aq)
aqueous hydrogen hypoiodite
Key Notes
Acid formulas must include (aq) to be named as acids. IUPAC is more systematic and includes physical state. Classical naming is more traditional and widely used in general chemistry.