factors effecting rate of reaction + collision theory
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
collision theory
for a chemical reaction to take place the particles need to collide with eachother with sufficient energy
rate of chemical reaction is
how fast reactants are used up OR how fast products are formed
concentration
if you increase the amount of particles in the same volume/space then your increasing frequent successful collisions as they are more likely to collide
temperature
increases particles internal energy they move faster and collide more frequently with more energy
surface area
increasing surface are to volume ration keeps same amount of volume but increases area the particles can collide on increasing frequent successful collisions
a catalyst is
a substance that speeds up a reaction without being used up or broken down or used up
how does a catalyst speed up reaction
it offers a alternative energy pathway with a lower activation energy
activation energy is
the minimum amount of energy needed for a chemical reaction to start
rate of reaction =
reactants used up OR products formed/ time
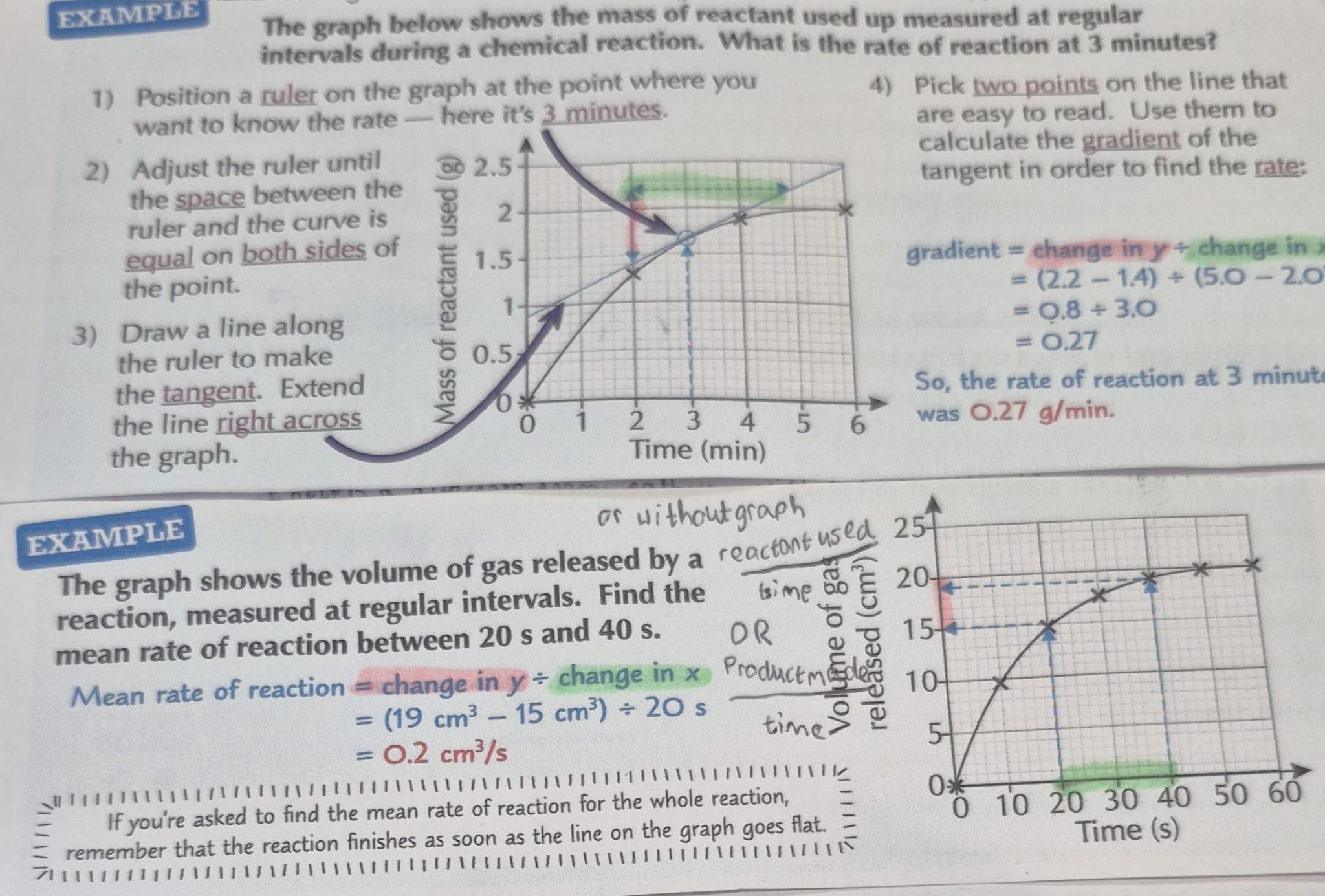
how to read rate of reaction off graphs
change in y/ change in x = gradient but also mean rate of reaction
tangent= precise rate at a point