Fuel cells
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
Fuel cells
Fuel cells is a type of galvanic cell that converts the chemical energy of the fuel into electrical energy. It can generate electricity as long as fuel is supplied to them.
Limitation of galvanic cells
Galvanic cell contain a small amount of reactants. Therefore, when the reactant has been consumed, the cell must be discarded resulting in waste; whereas in fuel cell the reactant are supplied continuously, allowing constant production of energy, therefore does not result in waste of the cell itself.
Similarities between fuel and galvanic cells
Involve a spontaneous redox reaction
Contain a negative anode and a positive cathode
Convert chemical energy from a fuel to electrical energy
include an electrolyte which allows the passage of ions
Includes an external circuit through which the electrons flow from the anode to the cathode.
Unlike Galvanic cells..
Rather than consuming a set of stored reactants, reactants must be continuously supplied to a fuel cell from an external source.
Fuel cells operate in open systems and usually involve gaseous reactants, whereas other galvanic cells usually operate in closed environment can cannot handle gaseous products.
Advantages of Fuel cells
Fuel cells convert chemical energy directly to electical energy. This is more efficient than the series of energy conversion that take place in fossil fuel power station: chemical energy to heat energy, to mechanical energy, to electrical energy, thus more energy efficient.
This means fuel is needed, reducing overall greenhouse gas emissions
It is a renewable energy source as it uses fuels like hydrogen which are readily available around us.
Hydrogen fuel cells produce water and heat as by products when hydrogen is used as fuel; no greenhouse gases, such as carbon dioxide are released.
FUel cells generate electricity as long as the fuel is supplied; convential batteries need to be recharged or replaced
Fuel cells generate electricity as long as the fuel is supplied; convential batteries need to be recharged or replaced
Fuel calls can use a variety of fuels
Disadvantages of Fuel cells.
Fuel cells required a contant fuel supply
Fuel cells are expensive as they are still a developing technology.
SOme types of cells use expensive expesnive electrolytes and catalysts
The use of fuel cells in transport requires an extensive network of hydrogen filing stations before it can become widespread
The hydrogen used in many fuel cells is mainly sourced from fossil fuels at present with energy losses and greenhouse gas emissions.
Difficulty of storing gaseous reactants
Gaseous reactants are odourless and colourless whilst being highly flammable compared to liquid fuel thus act as a safety hazard
Porous electrodes
Function of electrode is the site where oxidation and reduction occurs as well as contains catalyse that increase reaction rate.
Allow reactant gases to efficiently diffuse through them and come into contact with the electrolyte
The high surface area created by many tiny pores also maximises the effectiveness of catalyst increasing the reaction rate and hence further improving efficiency.
Electrolyte in fuel cells
Carries the ions from one electrode to anther to maintain electrical neutrality, to complete internal circuit and allow reaction to occur.
Must mention specific ions
Safety concerns
As hydrogen is highly flammable (can ignite easily when contact with flames) therefore when a leak occur, cannot be detected to other fuels such as ethanol, posing a safety risk.
This can be prevented with sturdy containners for storage of hydrogen appropriately in sutiable temperature and pressure.
Hydrogen is colorless, odorless, and leaks easily due to its small molecular size.
Specialized sensors are needed to detect leaks since hydrogen dissipates quickly.
Fuel cells generate electricity and water, but improper handling can lead to electrical hazards.
Short circuits or malfunctions can result in overheating or fire risks resulting in burns.
Improving efficicieny of fuel cells
Scientist can increase surface area of electrode by being porous where The high surface area created by pores also maximises the effectiveness of catalyst increasing the reaction rate and hence further improving efficiency.
Using electrodes that are catalytic to increase reaction rate
Using electrodes that are very good conductors to decrease heat lost through resistance of electrodes.
Difference between alkaline and hydrogen fuel cell
OH- ions are required at the anode for reaction with H2 in an alkaline cell but not in an acidic cell
Water is a reactant at the cathode in an alkaline cell. It is a product at the cathode in an acidic cell
Hydrogen ions travel from anode to cathode through the electrolyte in an acific cell. Hydroxide ions travel from cathode to anothe in an alkaline cell.
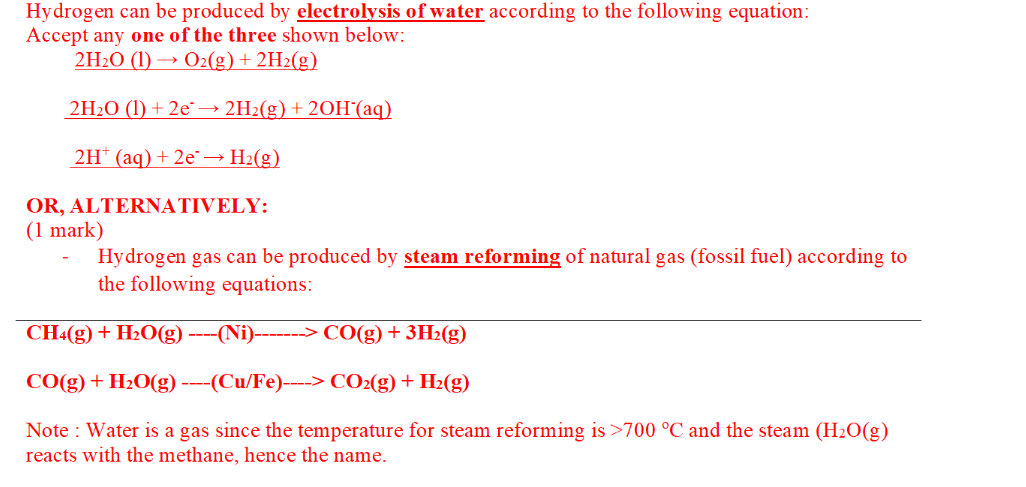
Steam reforming
Hydrogen can be produced by steam reforming of natural gas (fossil fuel) according to the following equations:
Steam reforming involves fossil fuel reacting with steam at high temperatures producing carbon monoxide and hydrogen gas.
Hydrogen produced by this method have lower energy content than original fuel and releases greenhouse gases
Linear economy
Operates on a ‘take-make-dispose’ model, making use of resources to produce products that will be discarded after use.
Circular economy
A continuous cycle that focuses on the optimal use and re-use of resources from the extraction of raw materials through to production of new materials, followed by consumption and re-purposing of unused and waste materials.