D2.3 Water Potential
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
Solvation
the interaction of a solvent (often water) with the dissolved solute. [simple: the interaction between solvent and solute molecules]
what is the most important factor in determining how well it solvates a particular solute?
Solvent polarity
what is osmosis?
the (ne)t movement of water through a semi-permeable membrane. moving from low solute and high water concentration to high solute and low water concentration. ( hypotonic → hypertonic )
water moves from less solute concentration to more solute concentration to make both sides equal. so water does this until it reaches equilibrium.
what is a hypertonic solution?
a solution that has high (more) solute and low (less) water concentration compared to another solution
what is a hypotonic solution?
a solution that has low (less) solute and high (more) water concentration compared to another solution
what is an isotonic solution?
a solution that has the same concentration of water and solute.
what does concentration mean?
the amount of solute (in moles) per unit volume (in dm3)
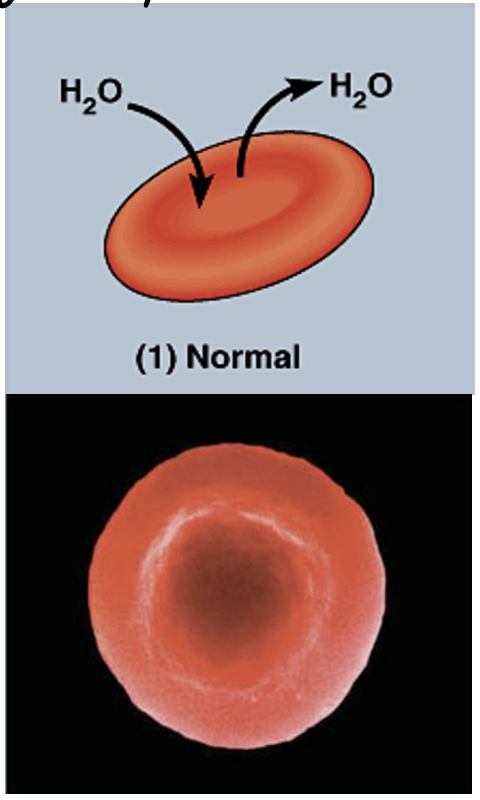
what is going on in this picture?
cell is in an isotonic solution (so it has the same amount of solute and water concentration as the cell)
so water moves in and out of the cell at equal rates.
net movement = 0
normal cell.
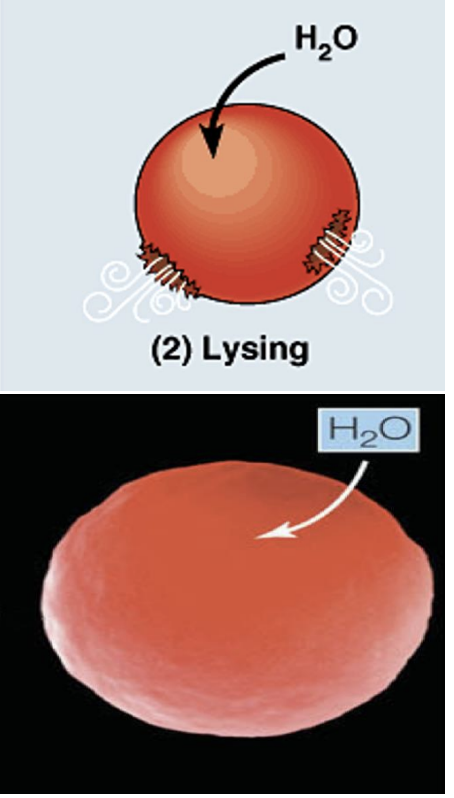
what is going on in this picture?
cell is in a hypotonic solution (so it has more solute and less water concentration than the cell)
so water moves out of the cell (water goes to area of high solute concentration)
this leads to low pressure in the cell, causing the cell to shrivels
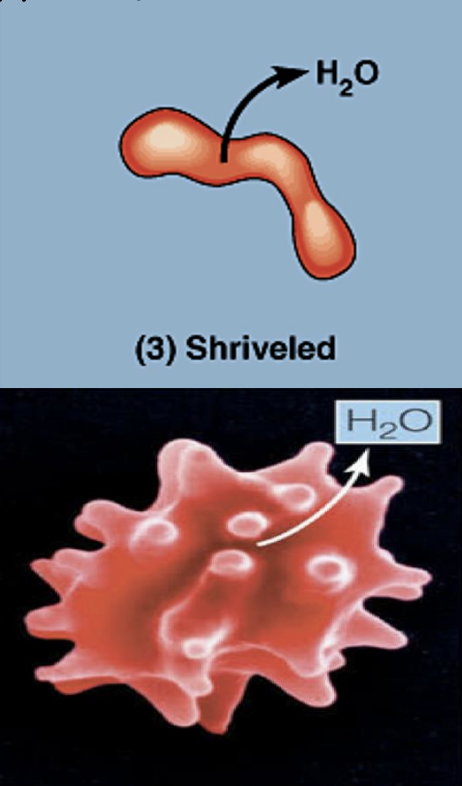
what is going on in this picture?
cell is in a hypertonic solution (so it has less solute and more water concentration than the cell)
so water moves into the cell (water goes to area of high solute concentration)
this leads to high pressure in the cell, causing the cell to burst / lyse (because it does not have (/lacks) a cell wall.)
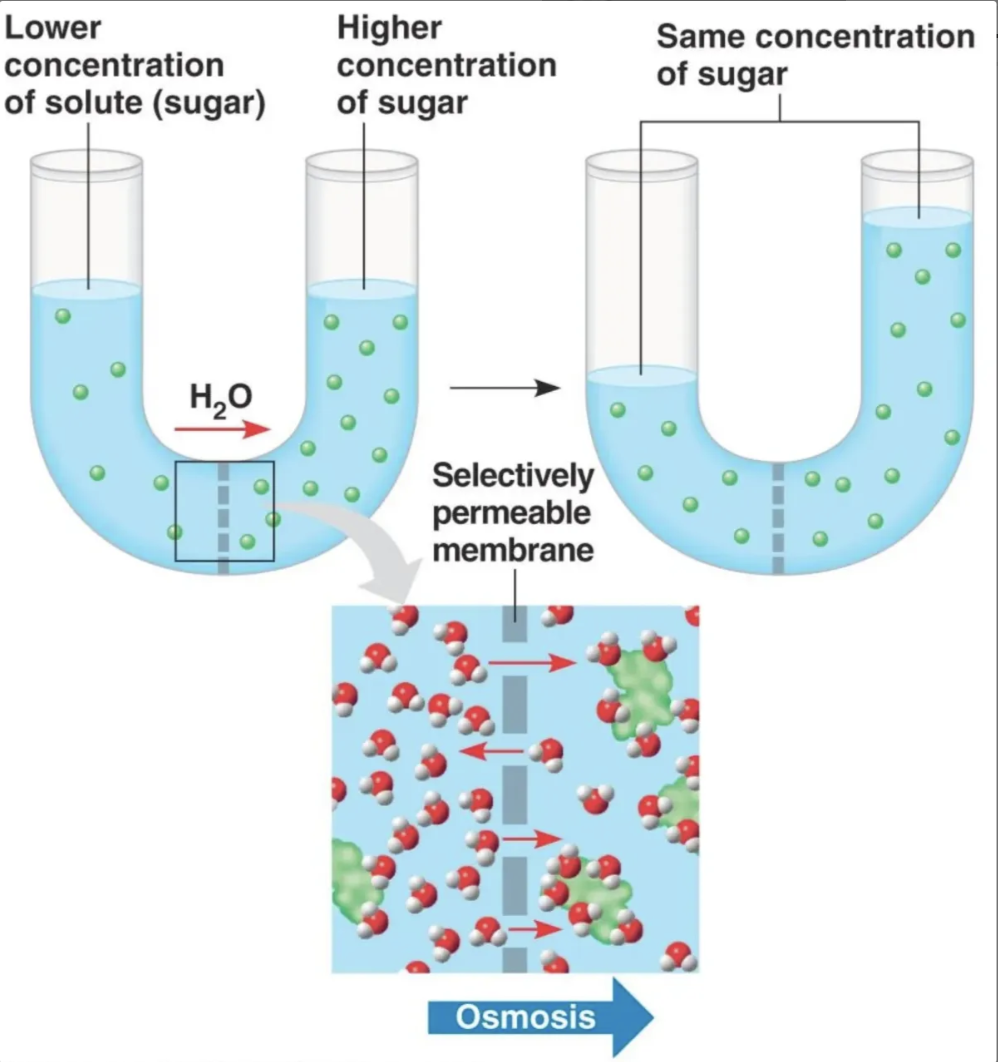
Can you explain why the water level changes?
osmosis is the movement of water (across a semipermeable membrane) from low solute concentration to high concentration. so water moved to the high solute concentration making water level rise. this is done till equilibrium is reached. after that the net movement is 0.
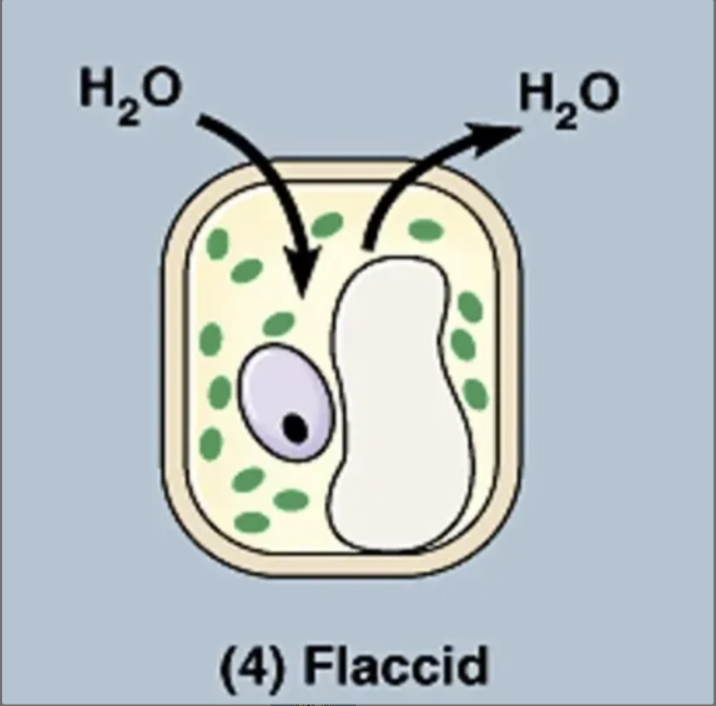
what is going on in this picture?
cell is in an isotonic solution (so it has the same amount of solute and water concentration as the cell)
so water moves in and out of the cell at equal rates.
net movement 0
normal plant cell (so the cell is flaccid).
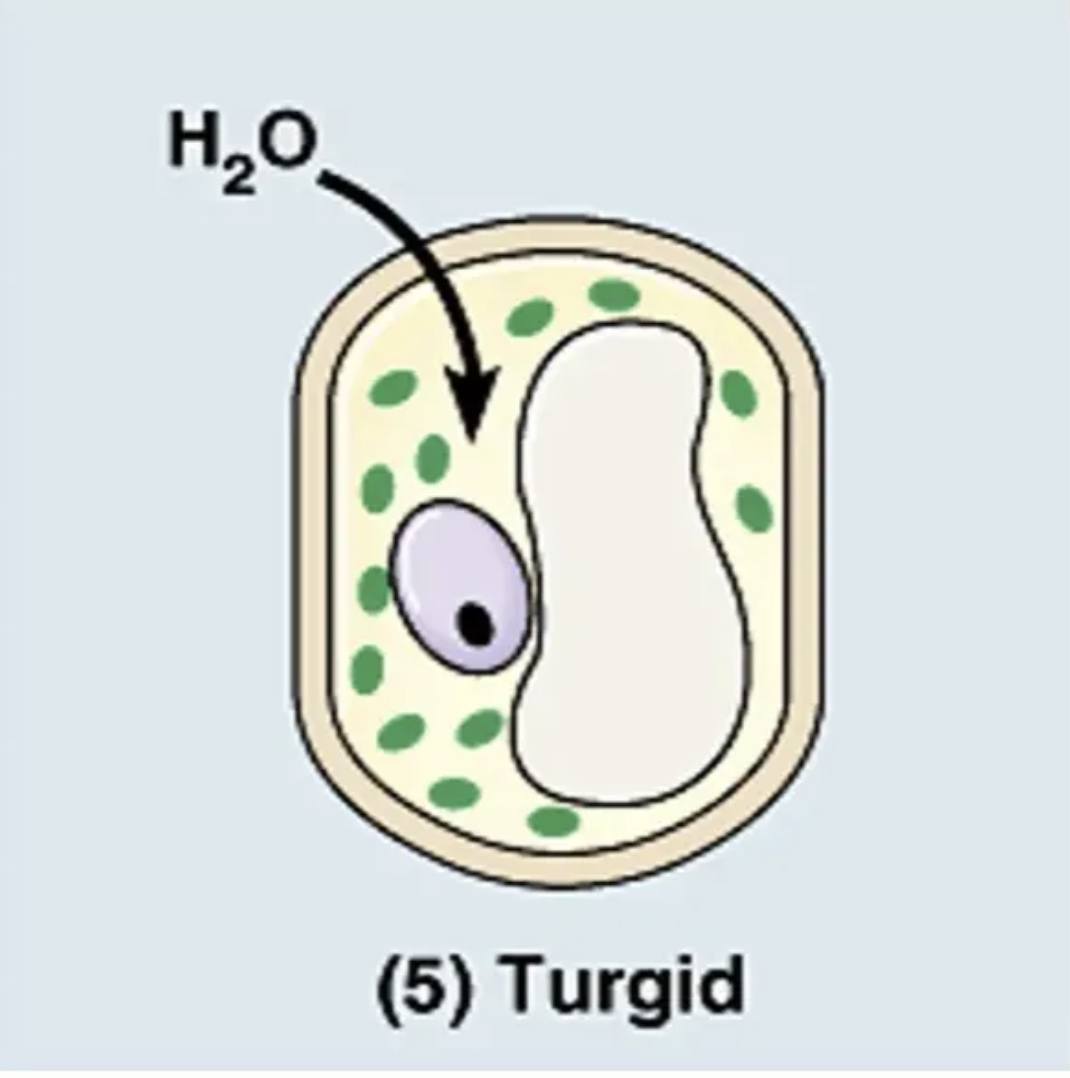
what is going on in this picture?
cell is in a hypertonic solution (so it has less solute and more water concentration than the cell)
making water move into the cell (water goes to area of high solute concentration)
★ the cell becomes turgid. but it maintains its shape because of its cell wall
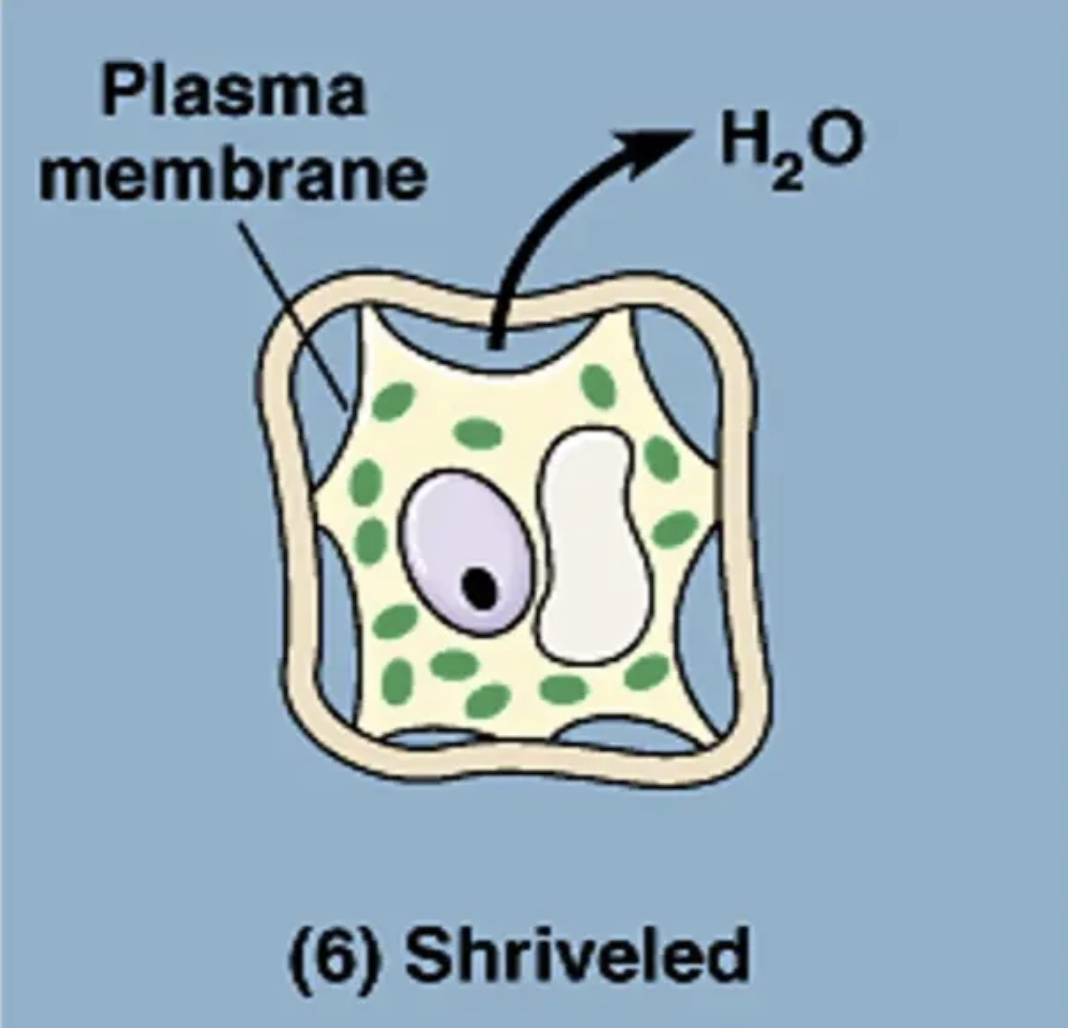
what is going on in this picture?
cell is in a hypotonic solution (so it has more solute and less water concentration than the cell)
so water moves out of the cell (water goes to area of high solute concentration)
★ because of the decreased pressure in the cell the plasma membrane pulls away from the cell wall, reducing cytoplasm volume.
this whole process is called: . ݁₊ ⊹ . ݁ plasmolysis ݁ . ⊹ ₊ ݁.
why do we use isotonic solutions for medical uses? give me examples of medical applications of isotonic solutions
Hypo- and hypertonic solutions can cause a lot of damage to cells. so doctors use isotonic solutions for medical uses, so the water in cells move in and out at equal rates.
examples: iv drops, organ preservative solution