Using concentration of solutions 2
1/3
Earn XP
Description and Tags
https://www.youtube.com/watch?v=Z93_atEmxNI&list=PL9IouNCPbCxUhxxFUbR4SNfwmaRB8mYX3&index=21&ab_channel=Freesciencelessons
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
4 Terms
How do you work out concentration is mol / dm³
Number of moles / volume (dm³)
Rearrange the equation of concentration to work out number of moles
concentration ( mol/dm³) x volume (dm³)
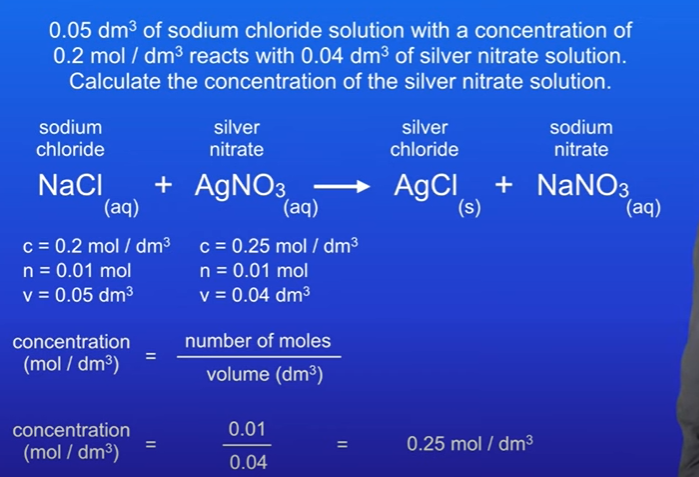
0.05 dm³ of sodium chloride solution with a concentration of 0.2 mol / dm³ reacts with 0.04 dm³ of silver nitrate solution, Calculate concentration of silver nitrate solution.
Concentration of sodium chloride is 0.2 mol / dm³
Volume of sodium chloride is 0.05 dm³
Volume of silver nitrate is 0.04 dm³
Work out number of moles for sodium chloride ; 0.2 × 0.05 = 0.01 moles.
We know that 1 mole of sodium chloride reacts with 1 mole of silver nitrate, therefore we must also have 0.01 moles of silver nitrate.
Calculate concentration of silver nitrate; 0.01 / 0.04 = 0.25 mol / dm³
a
a