CH302 Naming Organic Compounds Flashcards
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
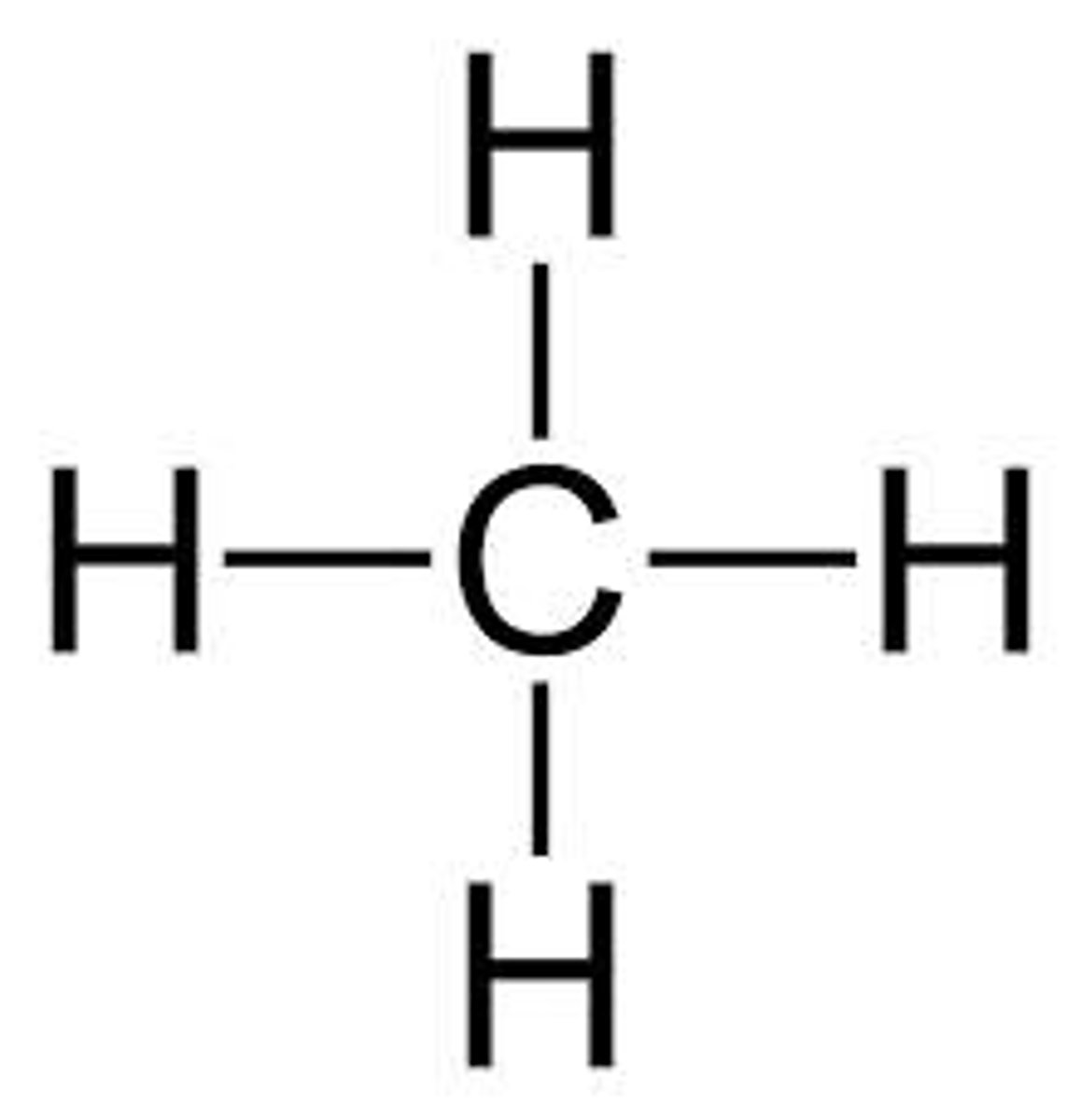
Given the formula below, what is the name of the compound?
CH₄
Methane (1 Carbon)
Given the formula below, what is the name of the compound?
C₂H₆
Ethane (2 Carbon)
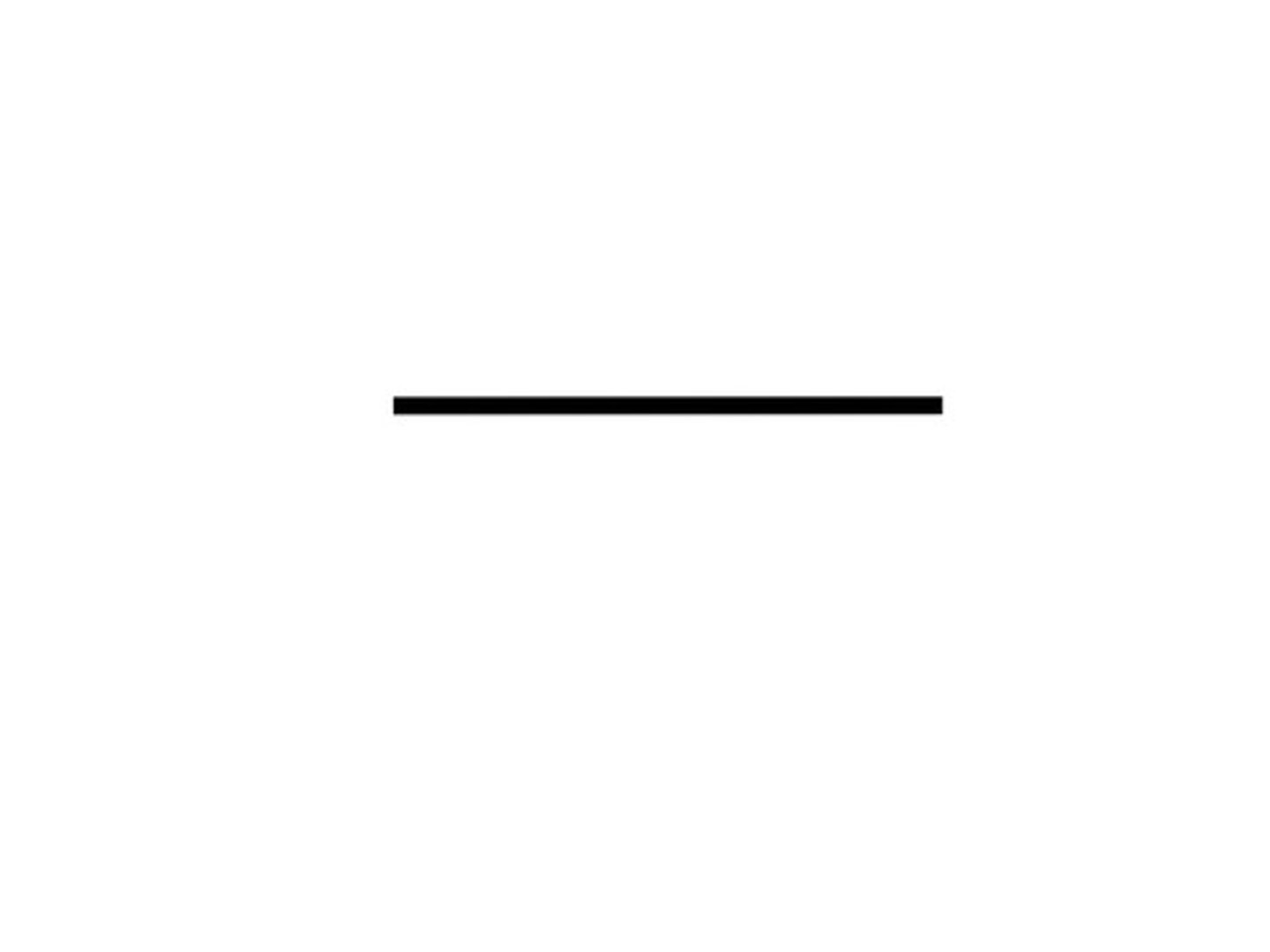
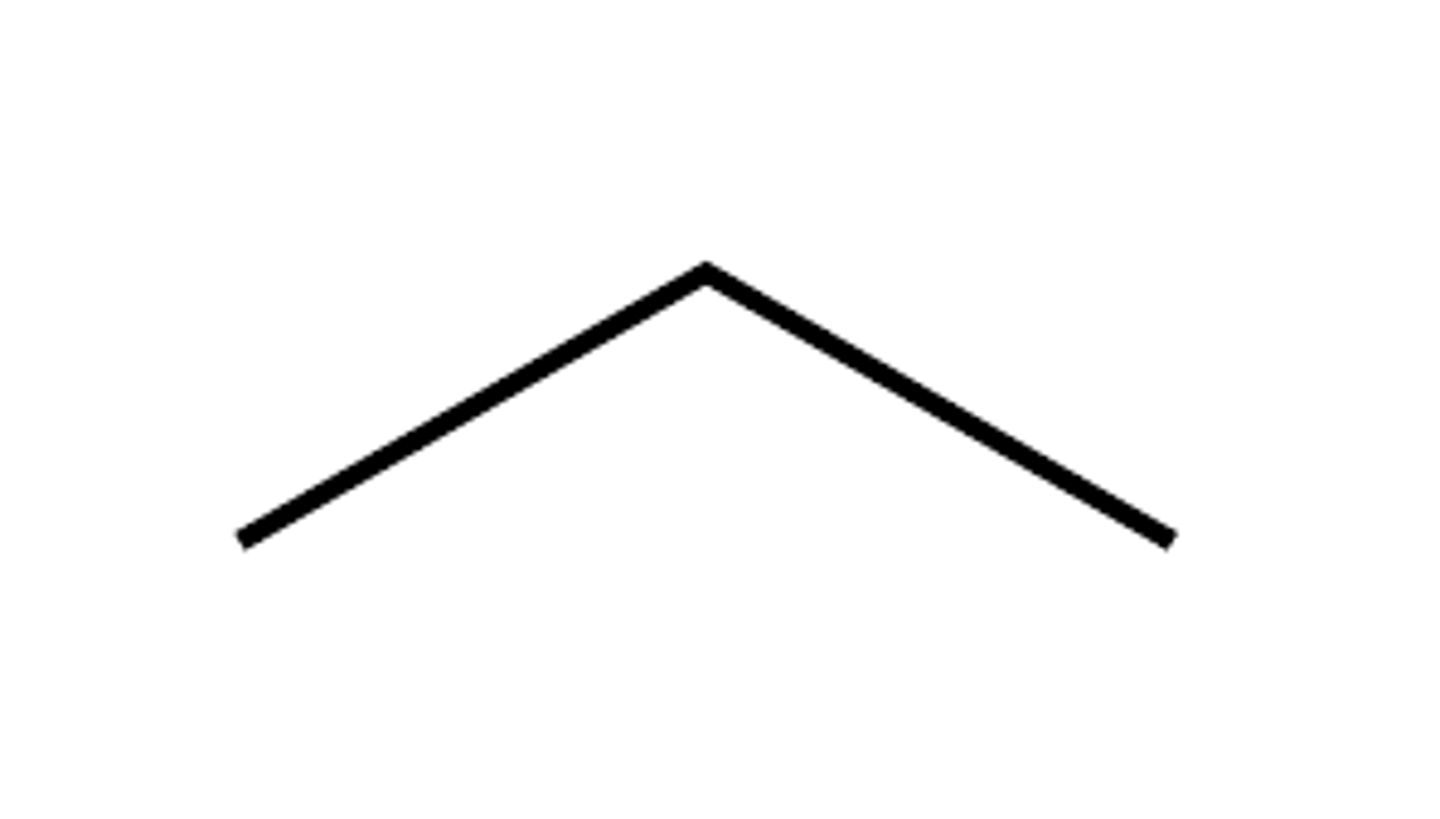
Given the formula below, what is the name of the compound?
C₃H₈
Propane (3 Carbons)
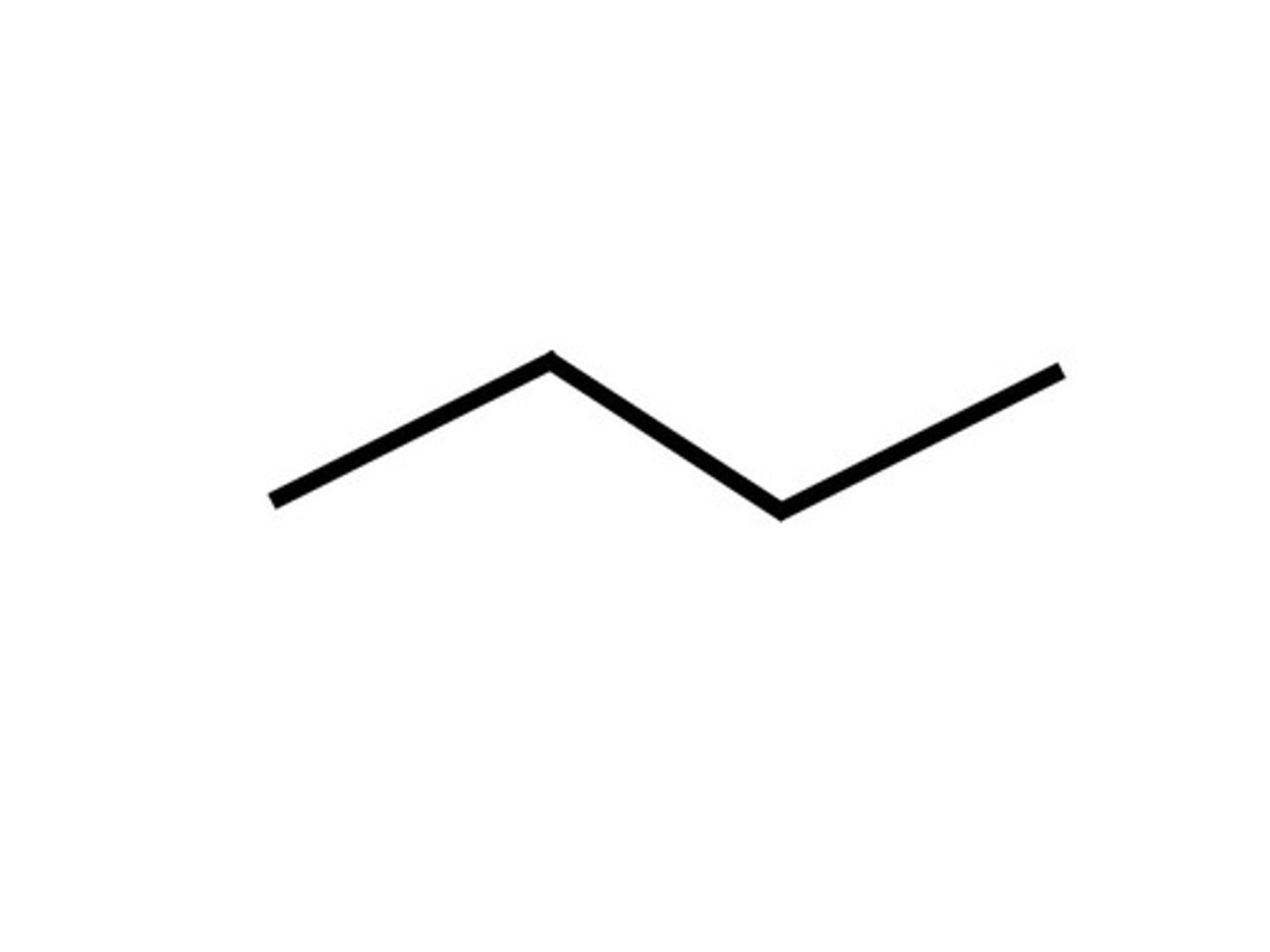
Given the formula below, what is the name of the compound?
C₄H₁₀
Butane (4 Carbons)
Given the formula below, what is the name of the compound?
C₅H₁₂
Pentane (5 Carbons)
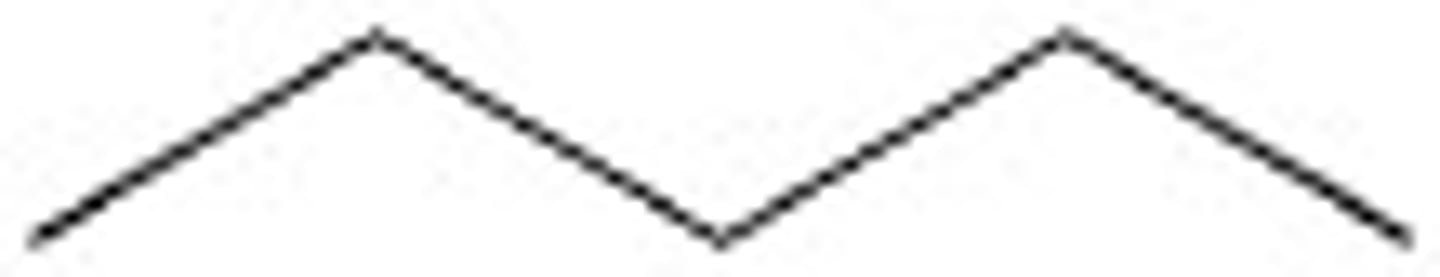
Given the formula below, what is the name of the compound?
C₆H₁₄
Hexane (6 Carbons)
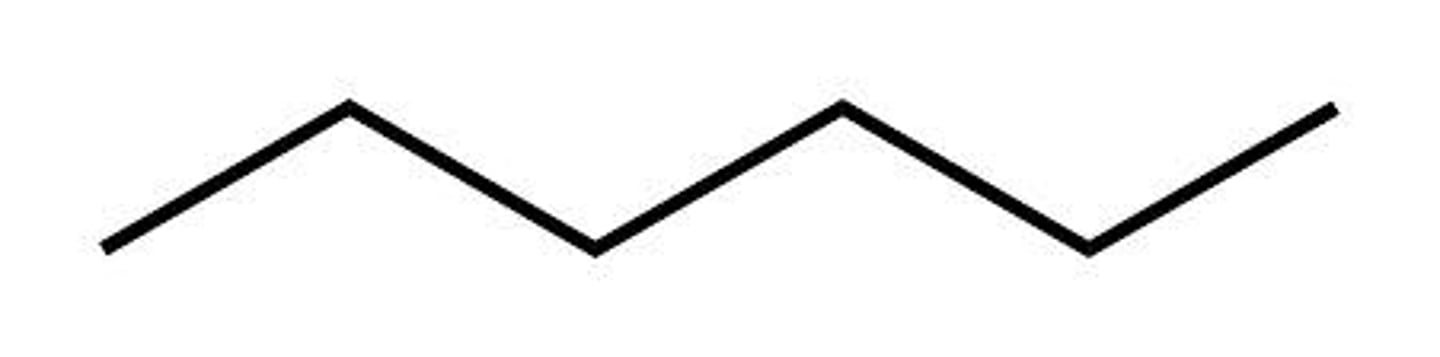
Given the formula below, what is the name of the compound?
C₇H₁₆
Heptane (7 Carbons)
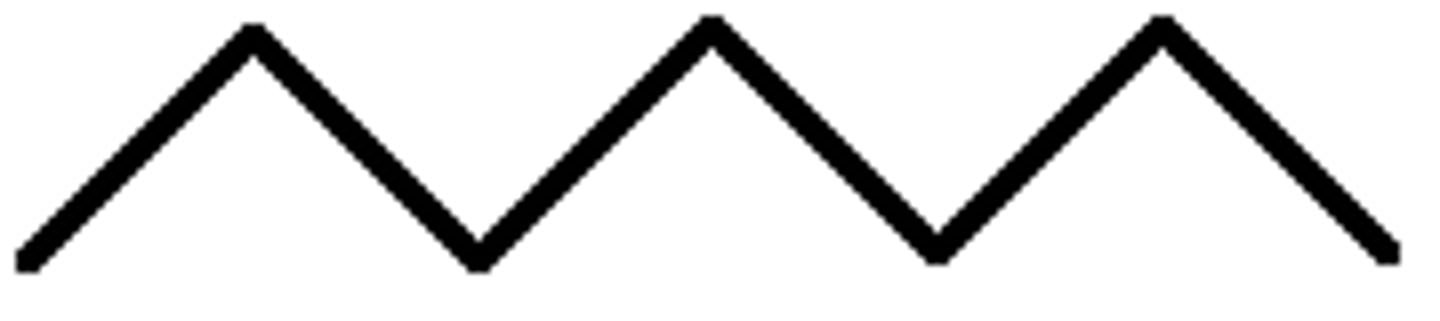
Given the formula below, what is the name of the compound?
C₈H₁₈
Octane (8 Carbons)

Given the formula below, what is the name of the compound?
C₉H₂₀
Nonane (9 Carbons)

Given the formula below, what is the name of the compound?
C₁₀H₂₂
Decane (10 Carbon)
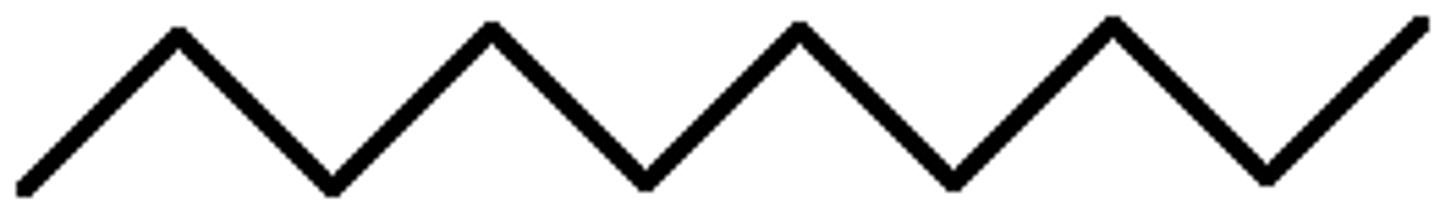
Alkane
A hydrocarbon containing only single bonds between carbon atoms.
Named with suffix -ane.
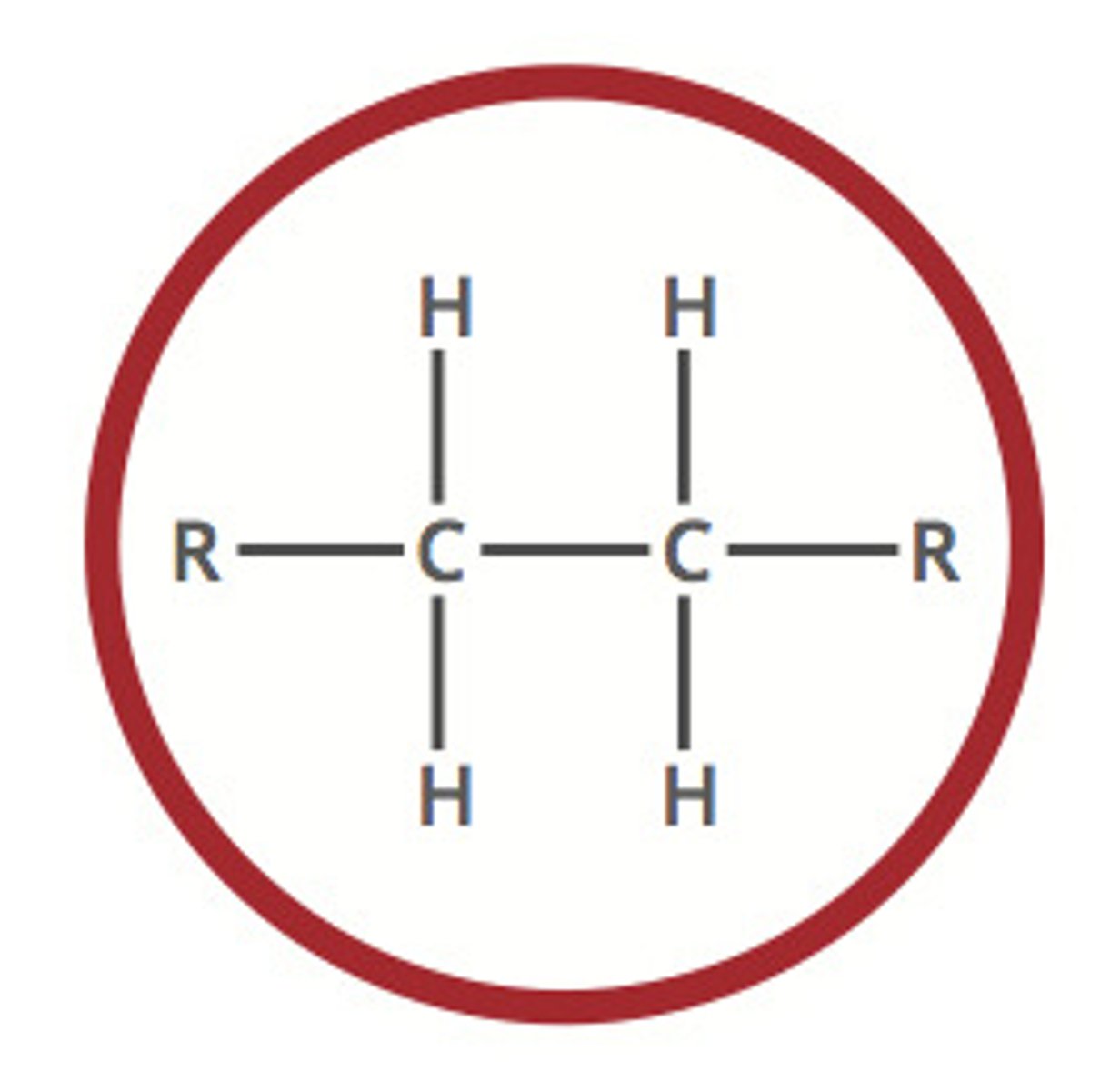
Describe a(n) Alkene
An hydrocarbon that contains at least one carbon-carbon double bond.
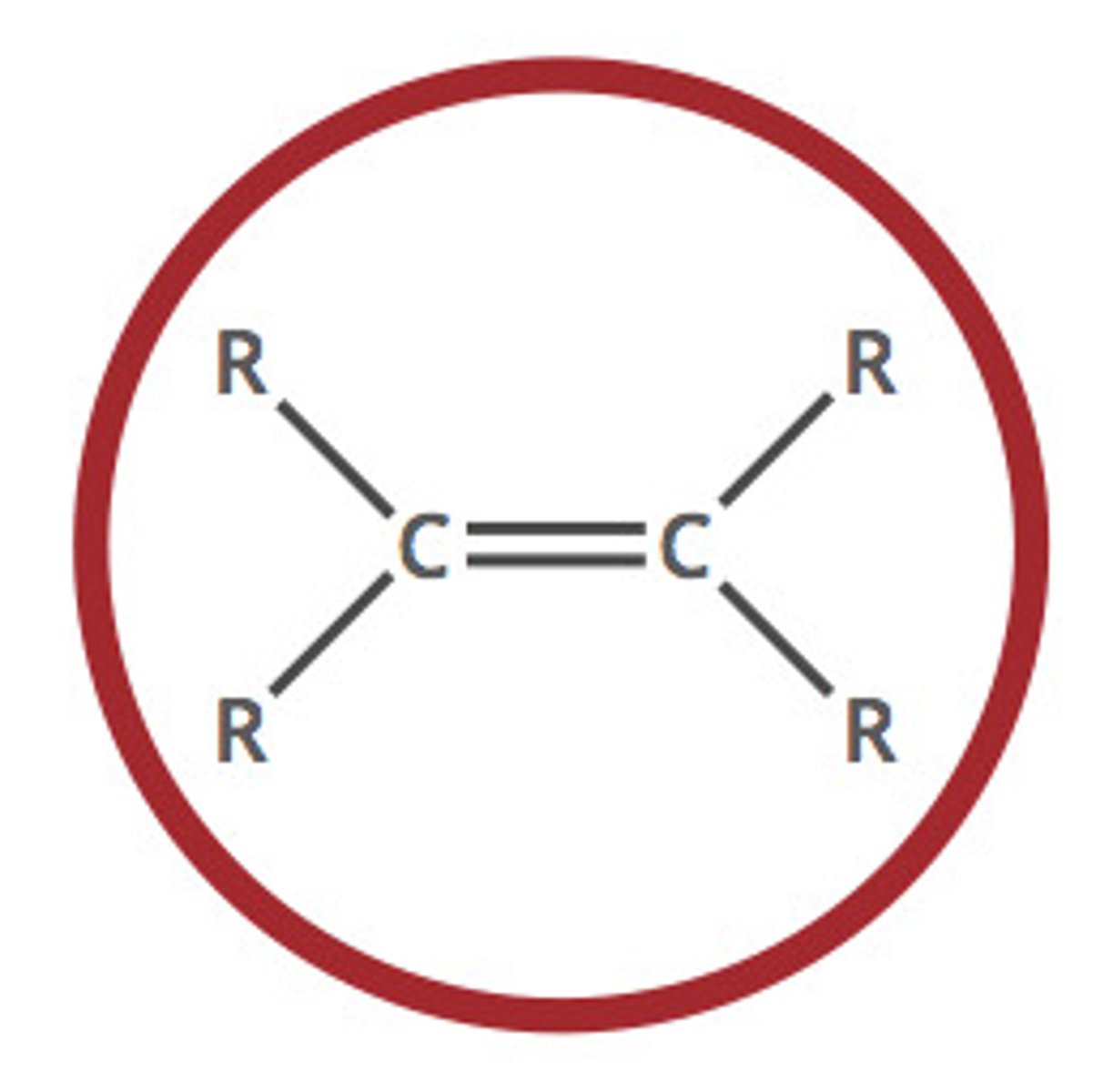
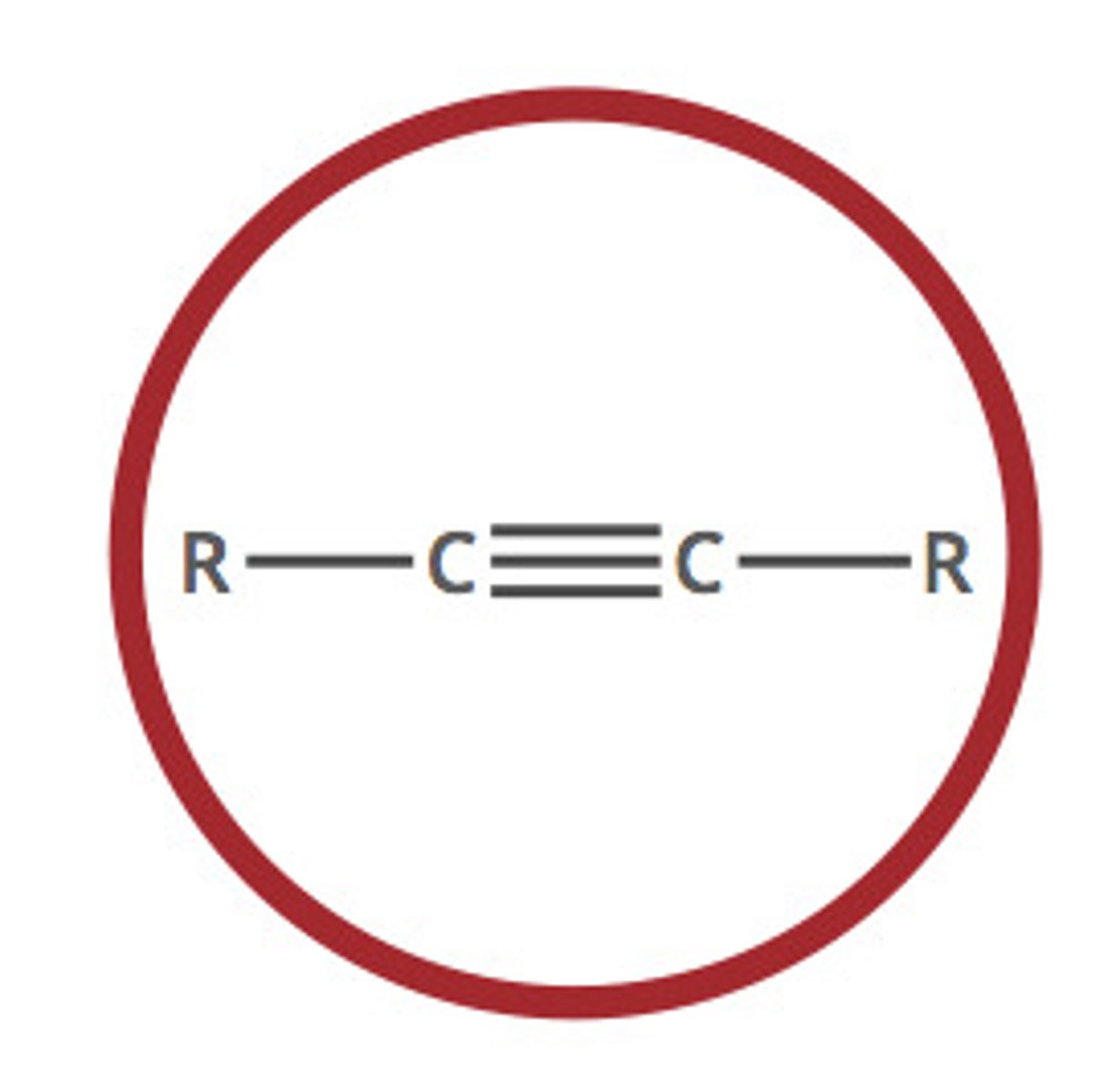
Describe a(n) Alkyne
An hydrocarbon characterized by at least one carbon-carbon triple bond.
Describe a(n) Thiol
A compound containing a sulfur atom bonded to a hydrogen atom and to a carbon atom, often represented as R-SH.
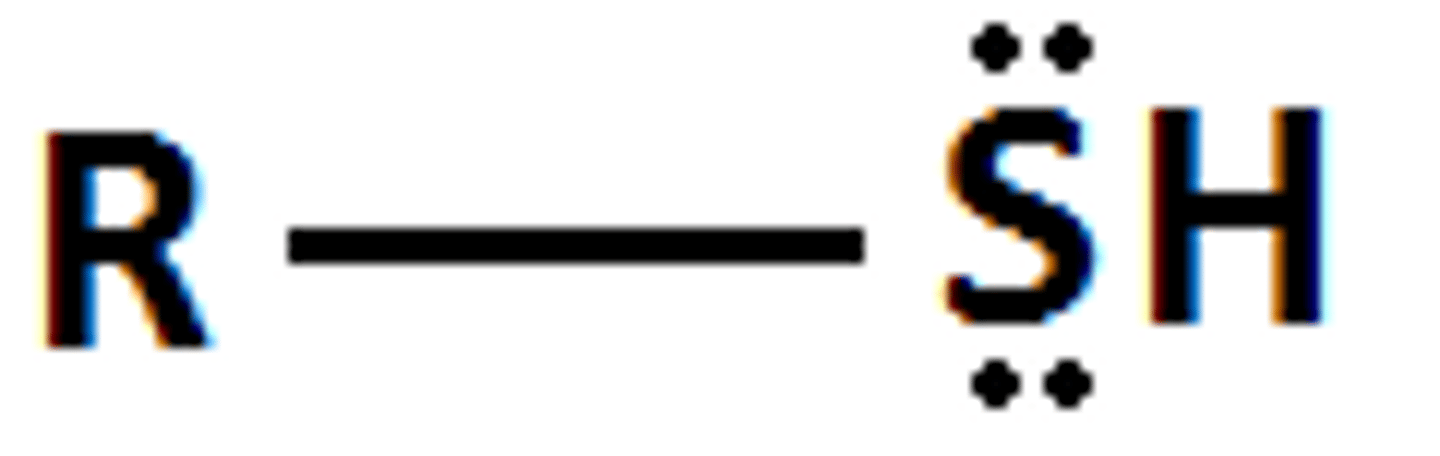
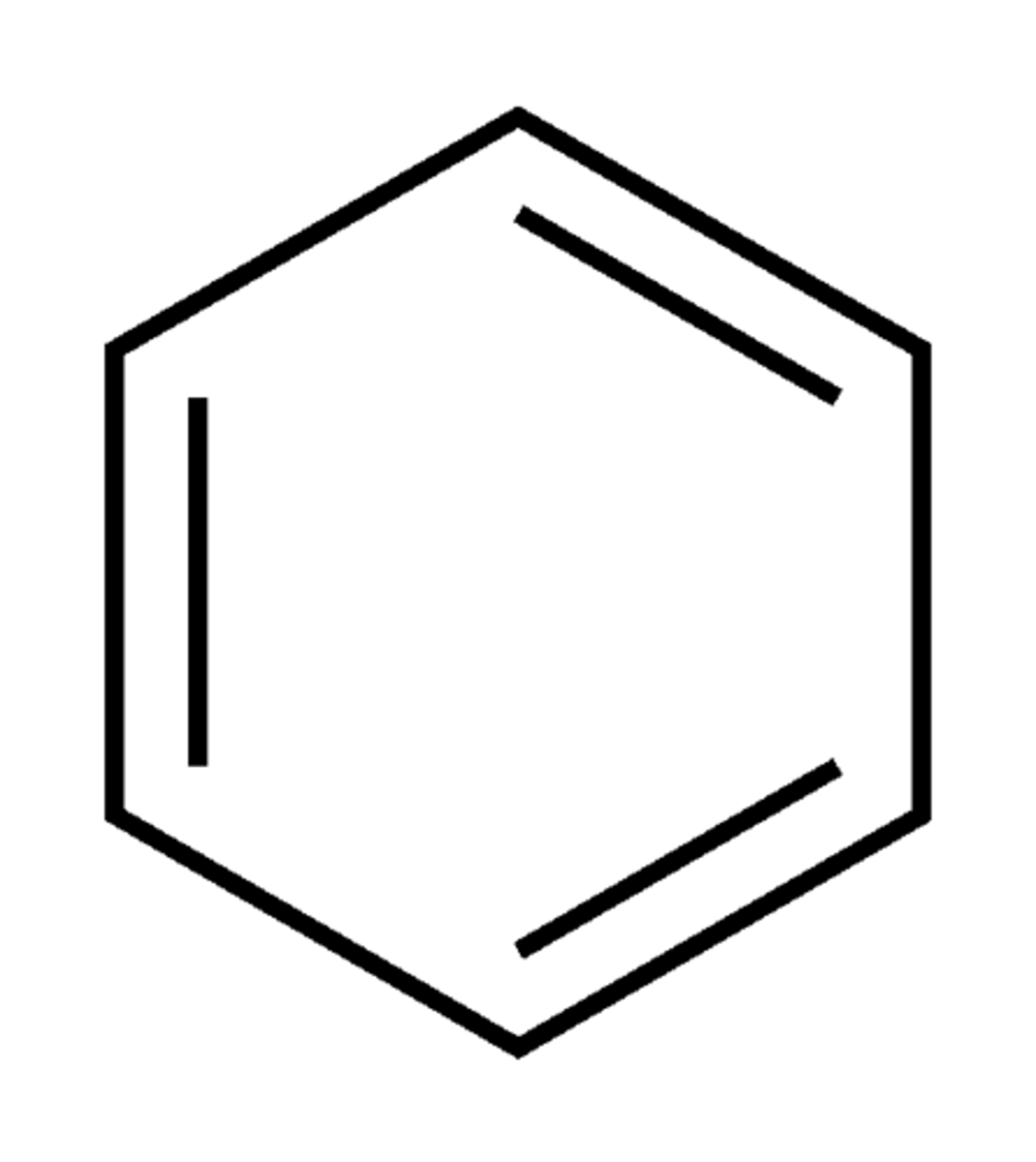
Describe a(n) Benzene ring
A cyclic structure consisting of six carbon atoms connected by alternating single and double bonds, known for its stability and aromatic properties.
Describe a(n) Alkyl halide
An organic compound containing a carbon atom bonded to a halogen atom (such as fluorine, chlorine, bromine, or iodine).
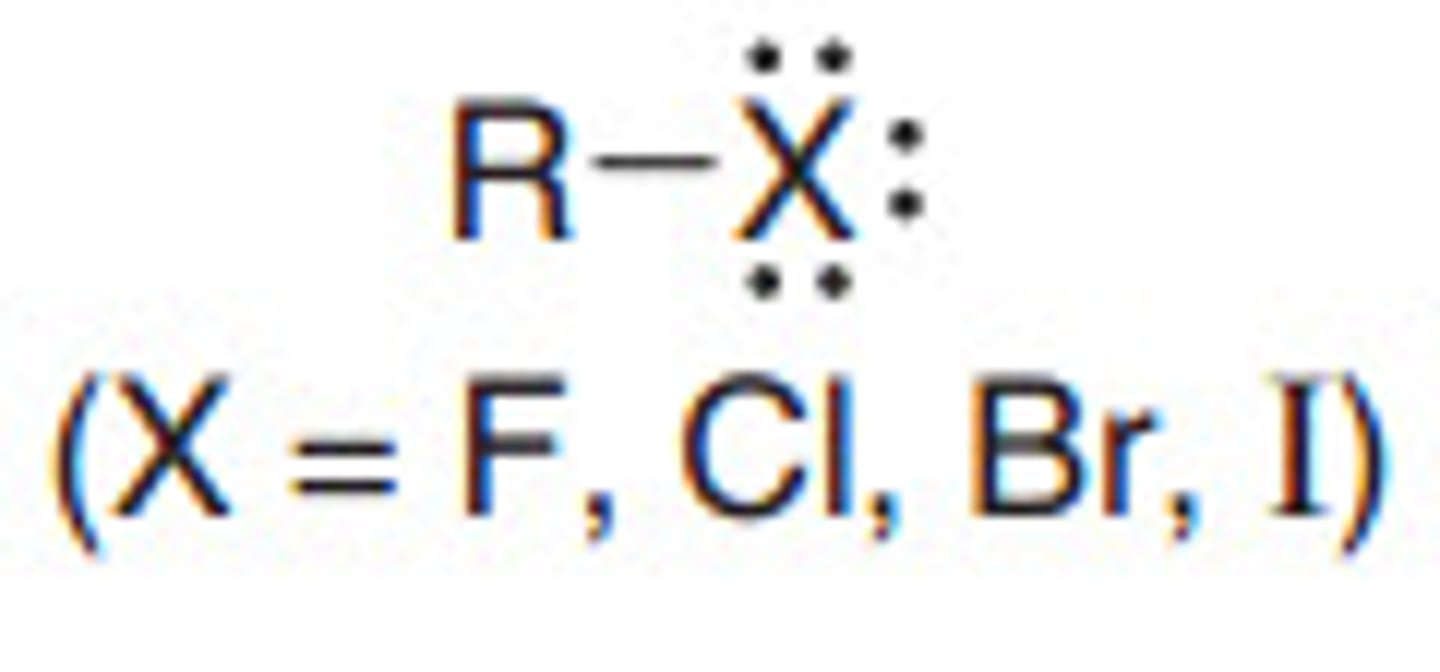
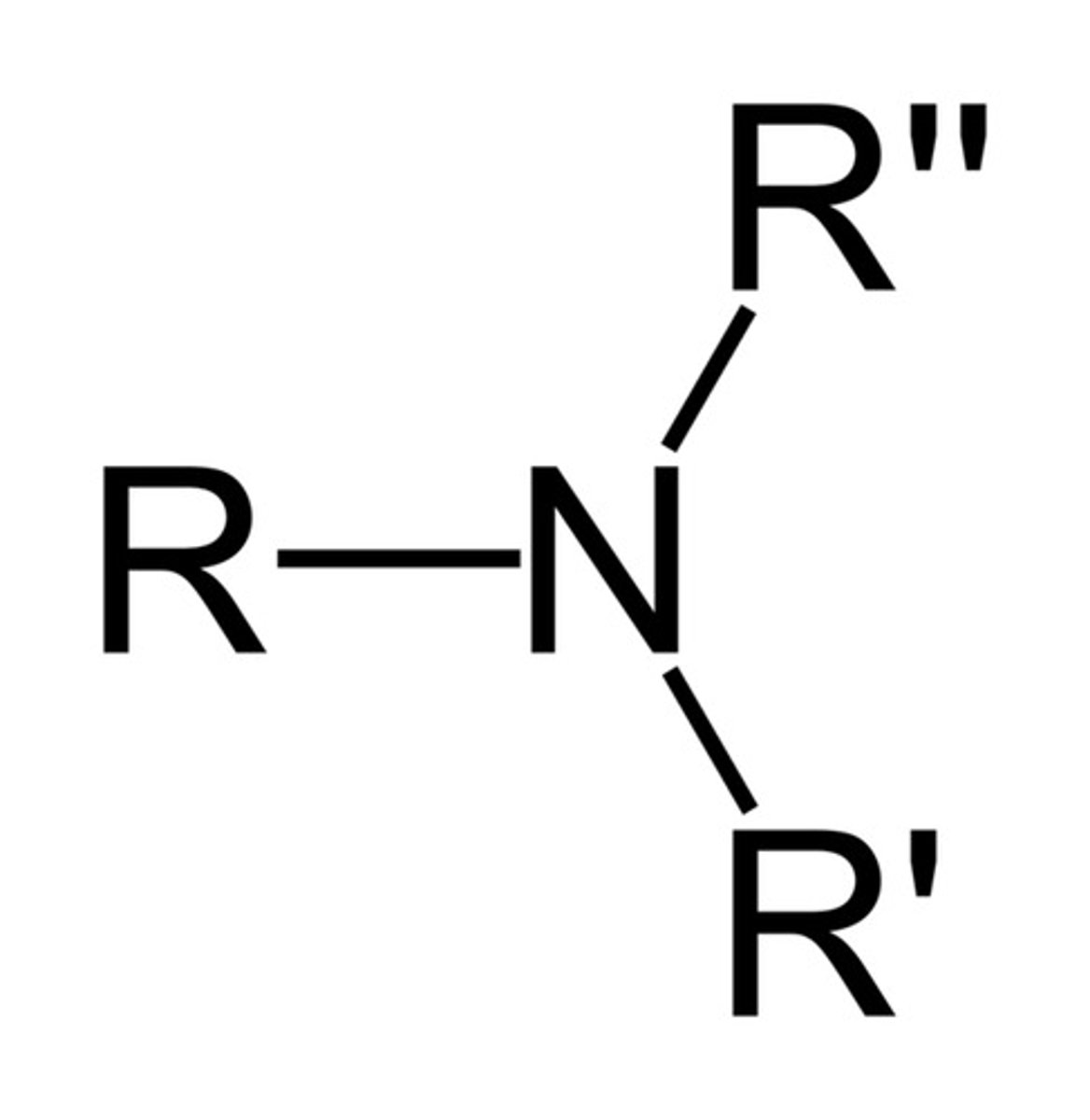
Describe a(n) Amine
An organic compound derived from ammonia (NH3) by replacing one or more hydrogen atoms with alkyl or aryl groups.
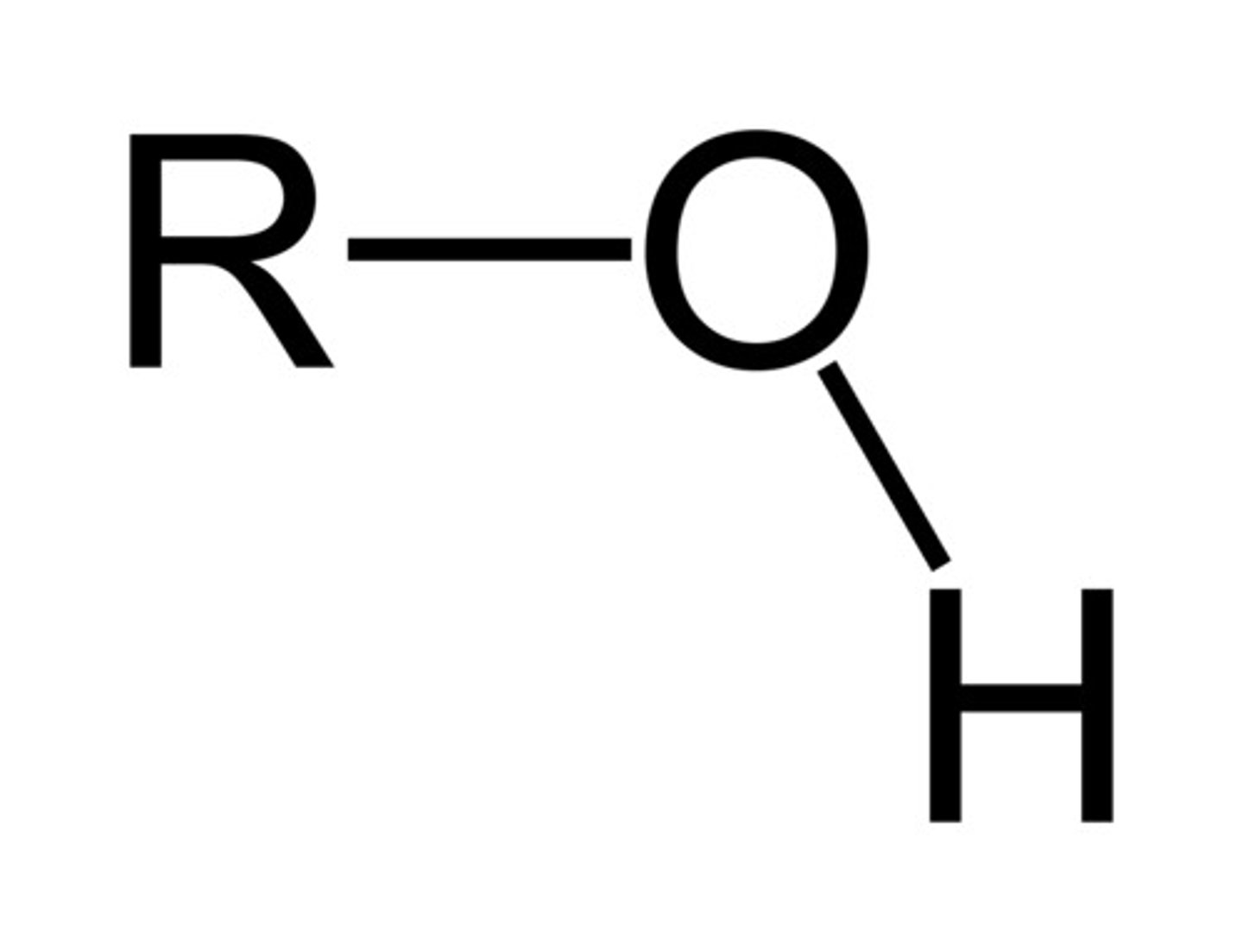
Describe a(n) Alcohol
An organic compound that contains one or more hydroxyl (-OH) groups attached to a carbon atom.
Describe a(n) Ether
An organic compound characterized by an oxygen atom bonded to two alkyl or aryl groups, typically represented as R-O-R'.
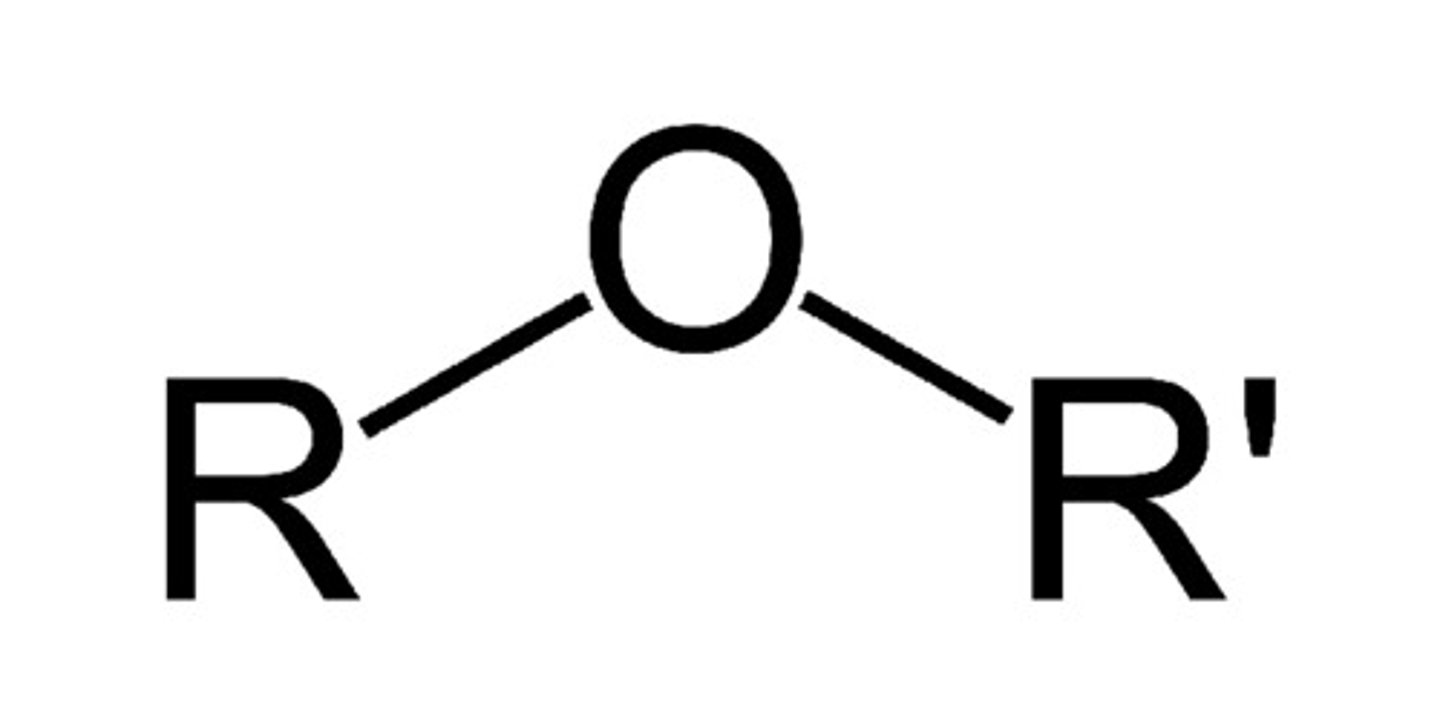
Describe a(n) Aldehyde
An organic compound containing a carbonyl group (C=O) with at least one hydrogen atom attached to the carbon atom of the carbonyl.
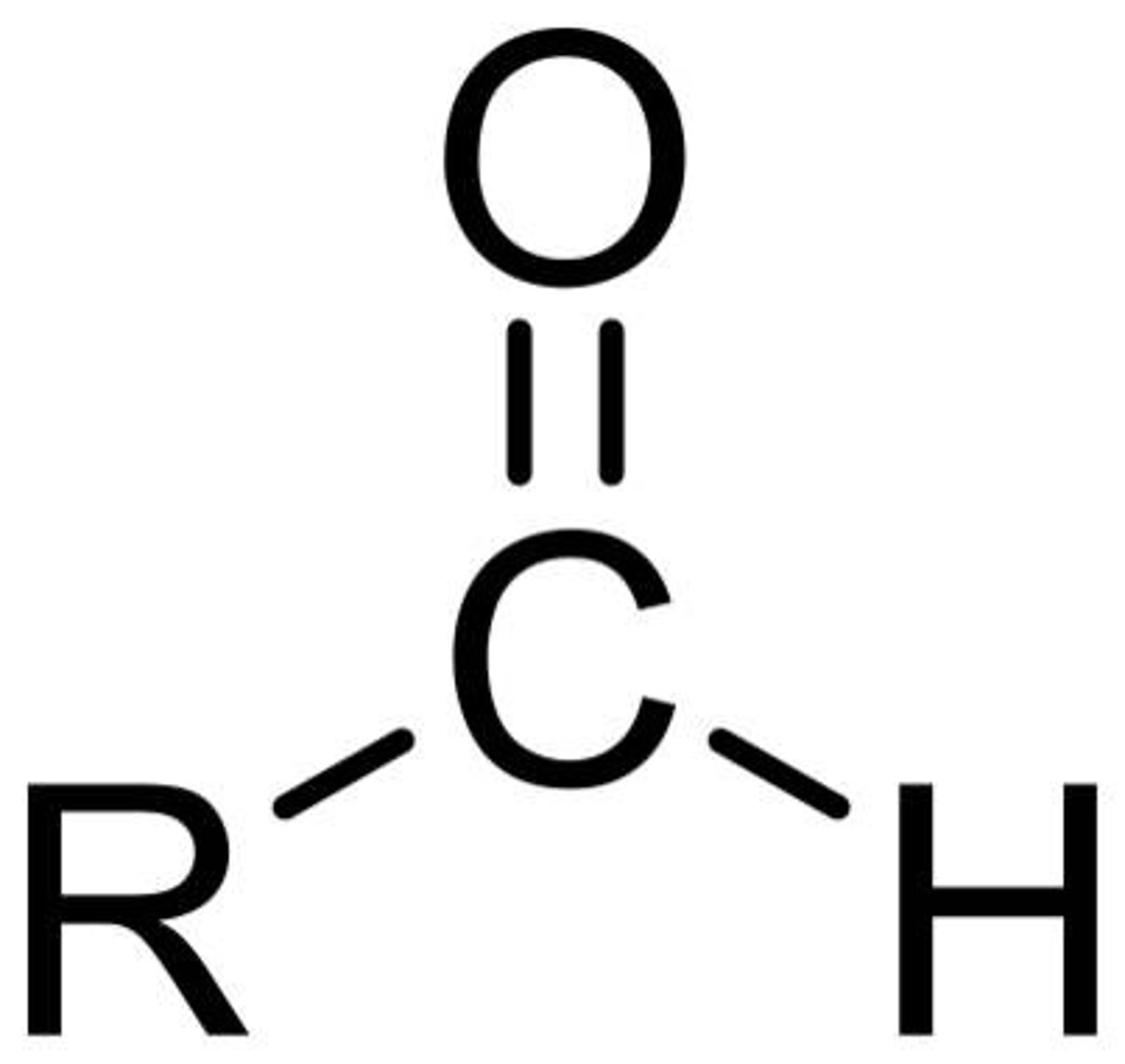
Describe a(n) Ketone
An organic compound containing a carbonyl group (C=O) bonded to two carbon atoms.
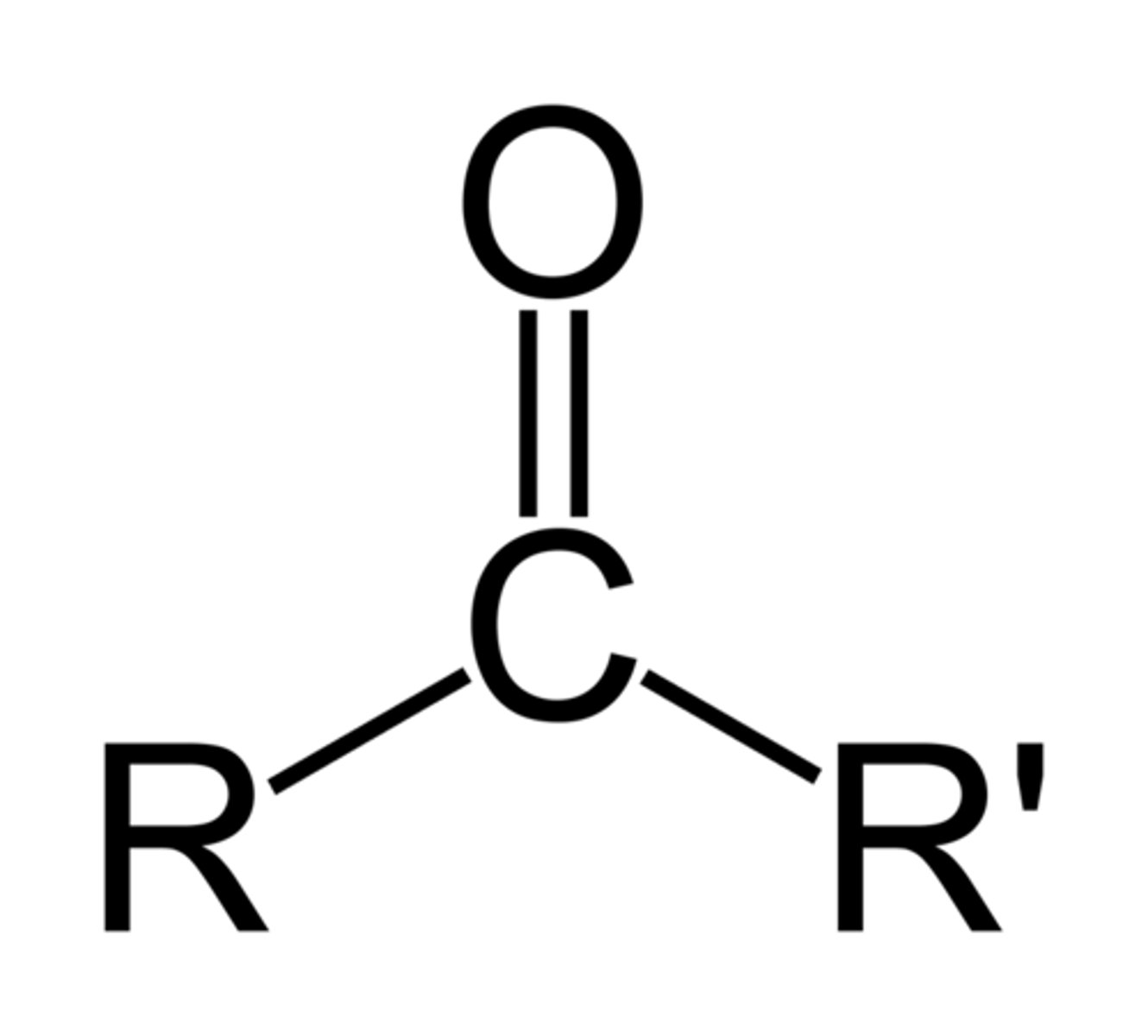
Describe a(n) Ester
An organic compound formed from the reaction of an alcohol and a carboxylic acid, characterized by the functional group RCOOR'.
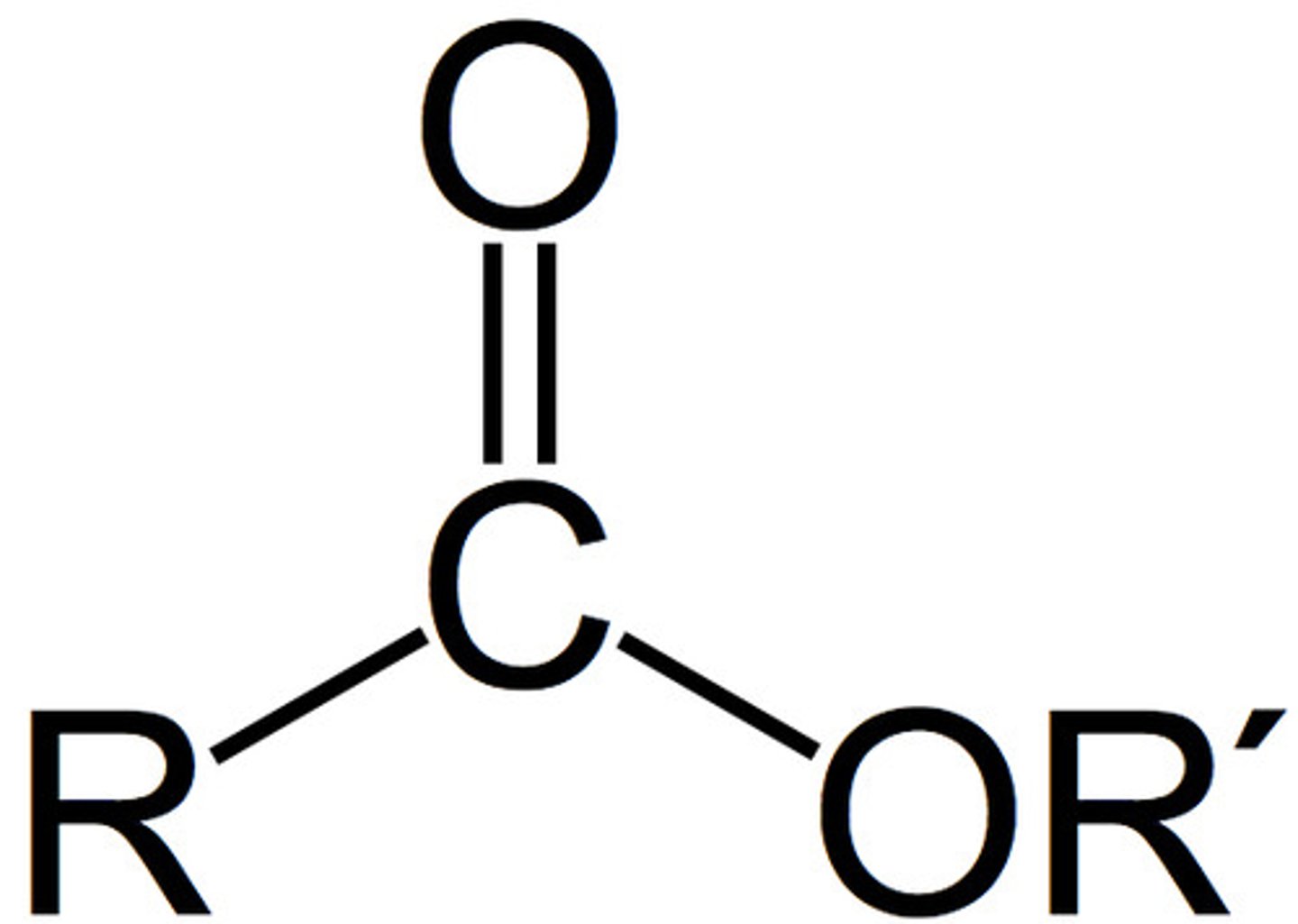
Describe a(n) Carboxylic acid
An organic compound containing a carboxyl group (-COOH), which consists of a carbonyl and a hydroxyl group.
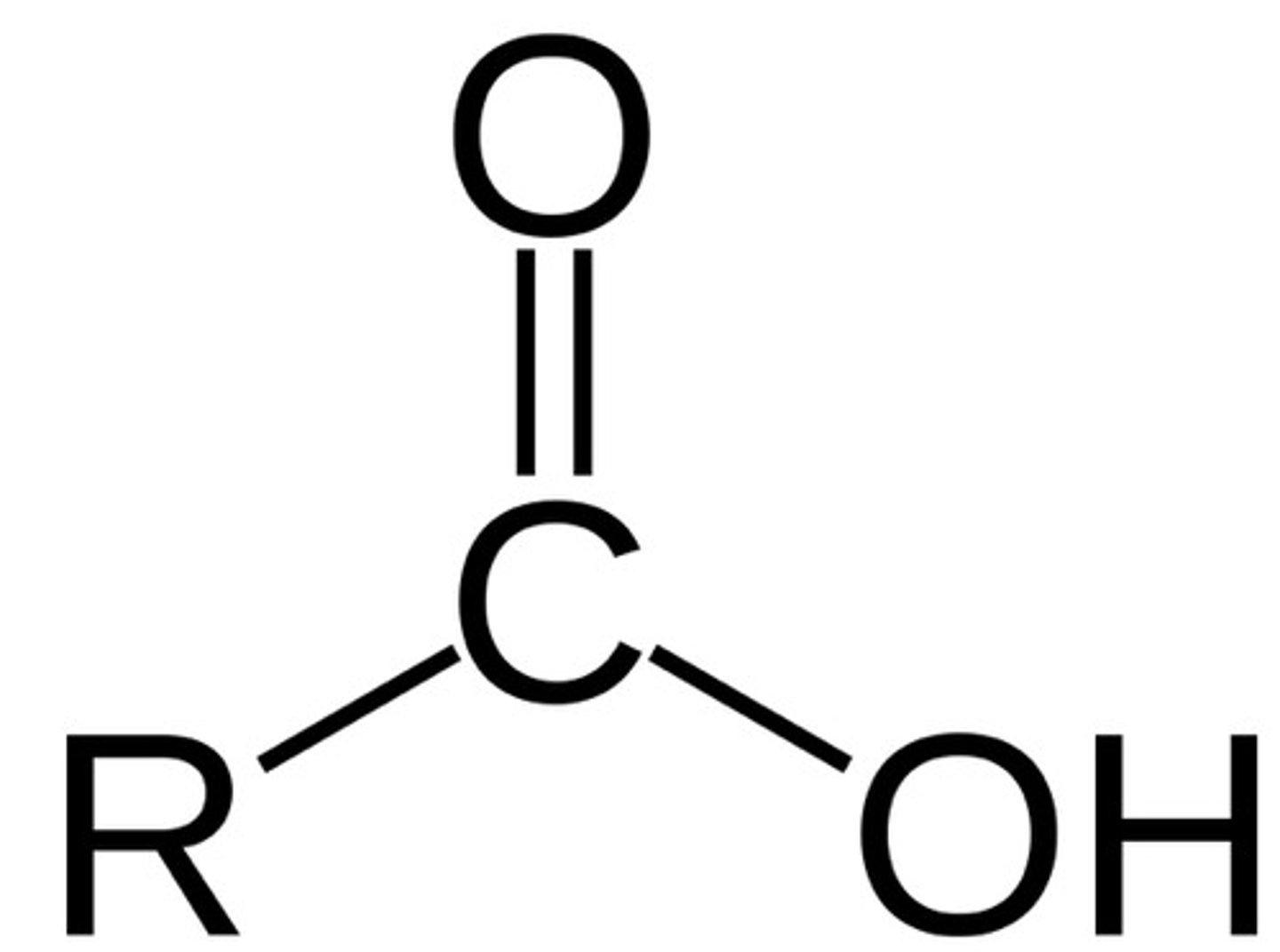
Describe a(n) Amide
An organic compound derived from a carboxylic acid where the hydroxyl group is replaced by an amine or ammonia.
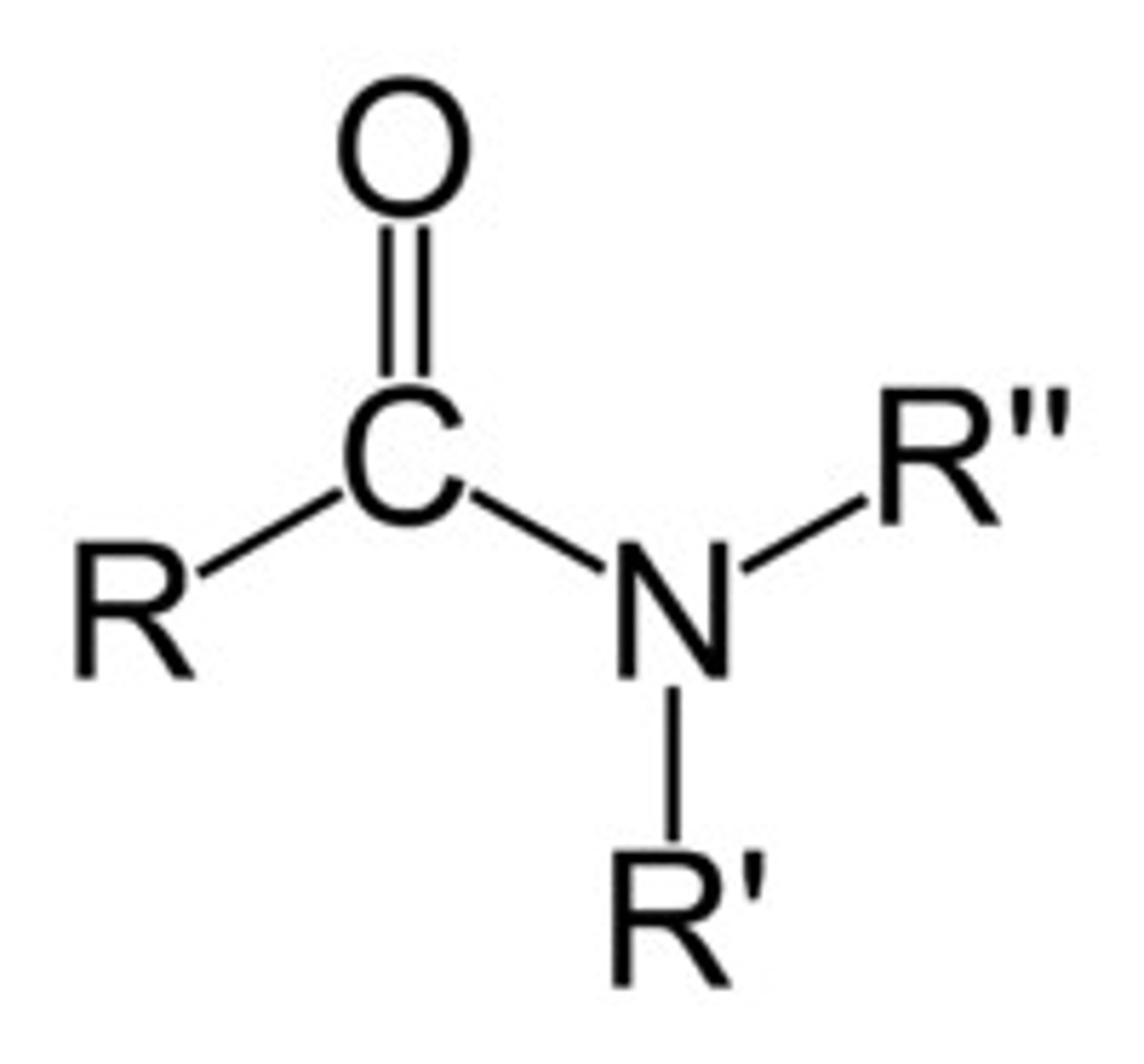
Describe a(n) NItrile
An organic compound containing a triple bond between carbon and nitrogen.
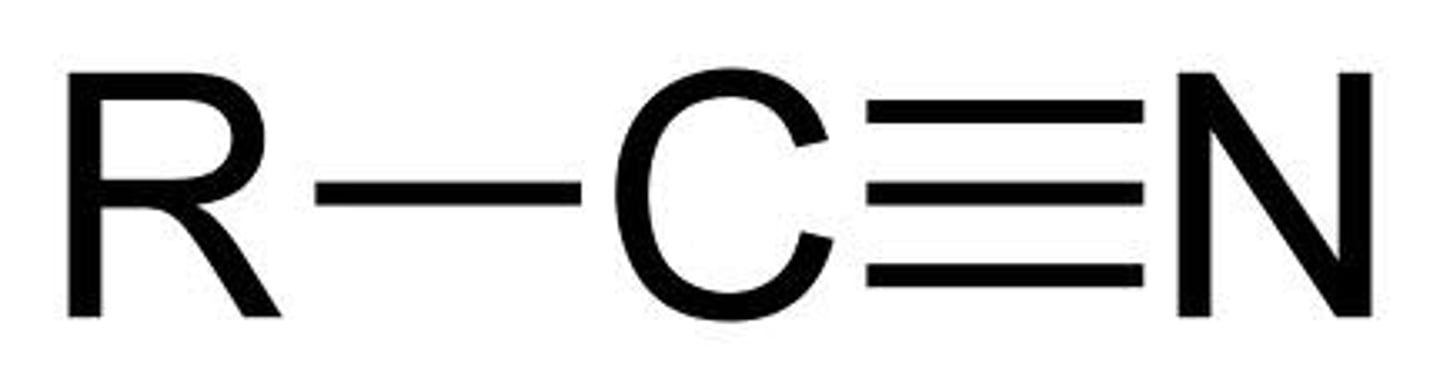
What is the naming convention of alkane?
-ane
What is the naming convention of alkene?
-ene
What is the naming convention of alkyne?
-yne
What is the naming convention of thiol?
-thiol
What is the naming convention of arene/benzene?
-benzene or -phenyl
What is the naming convention of alkyl halide?
floro-, chloro-, bromo-, iodo-
What is the naming convention of amine?
-amine or -amino
What is the naming convention of alcohol?
hydroxy- or -ol
What is the naming convention of ether?
methoxy- or -ether
What is the naming convention of aldehyde?
-al
What is the naming convention of ketone?
-one
What is the naming convention of ester?
-anoate or -ate
What is the naming convention of carboxylic acid?
-oic acid
What is the naming convention of amide?
-amide