Complex ions & redox
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
What is a complex ion?
ion formed by a central metal ion surrounded by ligands forming coordinate bonds
What is a ligand?
an atom or ion or molecule that can form a coordinate bond with a metal ion
What is a coordinate bond?
a bond formed when both electrons shared come from the same species
What are the 3 types of shapes formed in a complex ion?
Octahedral, tetrahedral, rare square planar
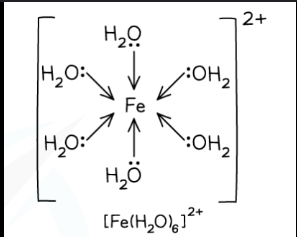
What is this?
Octahedral complex ion
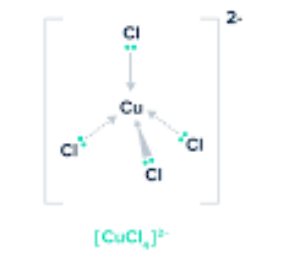
What is this?
Tetrahedral complex ion
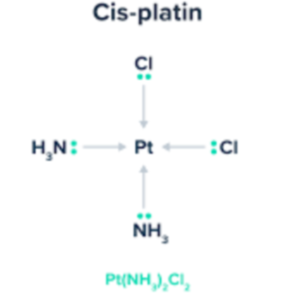
What is this?
Square planar complex ion
When does a octahedral complex ion become a tetarhedral ion?
When there’s more than 2 Cl- ligands as its too big for the octahedral
What are monodentates?
complex ions with 1 lone pair
Give examples of common monodentates:
H2O, CN-1, NH3, Cl-1, OH-1, ScN-1
What are bidentates?
complex ions with 2 lone pairs
Give common examples of bidentates:
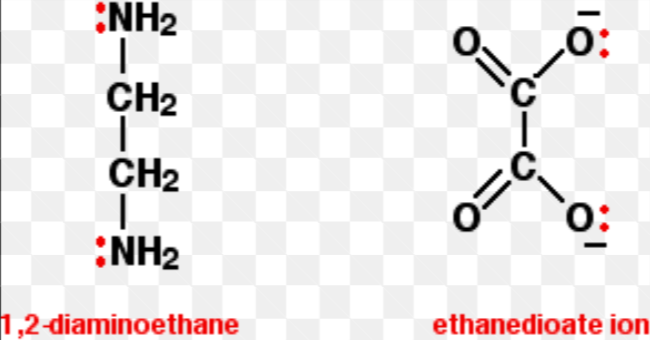
What are the interconversion equations between Fe3+ and Fe2+?
oxidation of Fe2+ → Fe3+ by Mn(VII) ions in acid solution
reduction of Fe3+ → Fe2+ by Iodide ions in solution
What are the interconversion equations between Cr3+ and Cr2O72-?
oxidation of Cr3+ to dichromate (VI) Cr2O72- using hydrogen peroxide
reduction of Cr2O72- to Cr3+ by acidified zinc
Give the reduction, oxoidation equations for oxidation of Cr3+ to dichromate Cr2O72- using hydrogen peroxide:
Reduction:
H2O2 +2e- → 2OH-
Oxidation:
2Cr(OH)6 3- + 4OH- → 2CrO4 2- + 8H2O + 6e-
Overall equation:
3H2O2 + 2Cr(OH)6 3- +4OH- → 6OH- + 2CrO4 2-+ 8H2O
Add acid:
CrO4 2- + 2H+ →Cr2O7 2- + H2O
What is the colour change when Cu2+ → Cu1+?
pale blue → colourless