Alkene/Alkyne Reactions and Synethesis
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
48 Terms
Start: Alkene
Reagents: H₂, Pd/Pt/Ni
Product: Alkane (syn addition)
Name: Catalytic Hydrogenation
Start: Alkene
Product: Alkane (syn addition)
Reagents: H₂, Pd/Pt/Ni
Name: Catalytic Hydrogenation
H₂, Pd/Pt/Ni
Start: Alkene
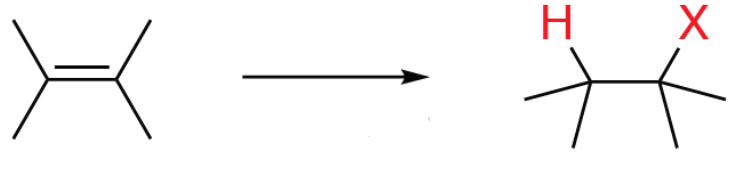
Reagents: HX (HCl, HBr, HI)
Product: Alkyl halide (Markovnikov)
Name: Hydrohalogenation
Start: Alkene
Product: Alkyl halide (Markovnikov)
Reagents: HX (HCl, HBr, HI)
Name: Hydrohalogenation
HX
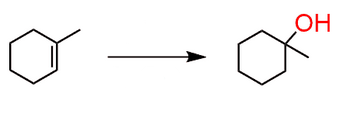
Start: Alkene
Reagents: H2O/H2SO4
Product: Alcohol (Markovnikov)
Name: Acid-Catalyzed Hydration
Start: Alkene
Product: Alcohol (Markovnikov)
Reagents: H2O/H2SO4
Name: Acid-Catalyzed Hydration
H2O/H2SO4
Start: Alkene
Reagents: 1) Hg(OAc)₂, H₂O 2) NaBH₄
Product: Alcohol (Markovnikov, NO rearrangements)
Name: Oxymercuration–Reduction
Start: Alkene
Product: Alcohol (Markovnikov, NO rearrangements)
Reagents: 1) Hg(OAc)₂, H₂O 2) NaBH₄
Name: Oxymercuration–Reduction
1) Hg(OAc)₂, H₂O 2) NaBH₄, NaOH
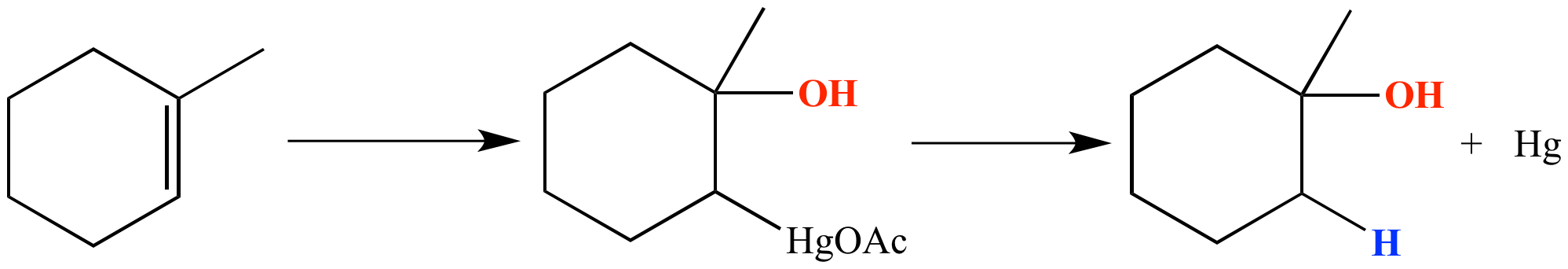
Start: Alkene
Reagents: 1) BH₃·THF 2) H₂O₂, OH⁻
Product: Alcohol (Anti-Markovnikov, syn)
Name: Hydroboration–Oxidation
Start: Alkene
Product: Alcohol (Anti-Markovnikov, syn)
Reagents: 1) BH₃·THF 2) H₂O₂, OH⁻
Name: Hydroboration–Oxidation
1) BH₃·THF 2) H₂O₂, OH⁻
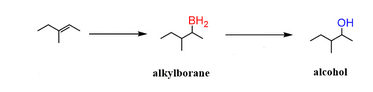
Start: Alkene
Reagents: X₂ (like Br₂ or Cl₂)
Product: Vicinal dihalide (anti)
Name: Halogenation


Start: Alkene
Product: Vicinal dihalide (anti)
Reagents: X₂ (like Br₂ or Cl₂)
Name: Halogenation
Br2
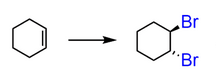
Start: Alkene
Reagents: Br₂ + H₂O
Product: Halohydrin (anti, OH on more substituted C)
Name: Halohydrin Formation
Start: Alkene
Product: Halohydrin (anti, OH on more substituted C)
Reagents: X₂ + H₂O
Name: Halohydrin Formation
X₂ + H₂O

Start: Alkene
Reagents: mCPBA
Product: Epoxide
Name: Epoxidation
Start: Alkene
Product: Epoxide
Reagents: mCPBA
Name: Epoxidation
mCPBA
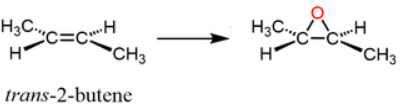
Start: Alkene
Reagents: OsO₄ (or cold KMnO₄)
Product: Vicinal syn diol
Name: Syn Dihydroxylation
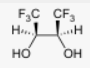
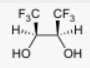
Start: Alkene
Product: Vicinal syn diol
Reagents: OsO₄ (or cold KMnO₄)
Name: Syn Dihydroxylation
OsO₄, H2S

Start: Alkene
Reagents: O₃ → (CH₃)₂S or Zn/H₂O
Product: Aldehydes/Ketones
Name: Ozonolysis
Start: Alkene
Product: Aldehydes/Ketones
Reagents: O₃ → (CH₃)₂S or Zn/H₂O
Name: Ozonolysis
1) O3, 2) H2O2
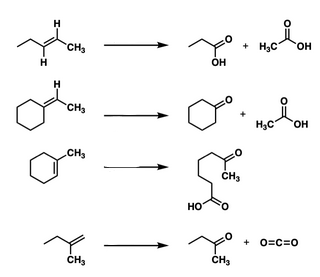
Start: Alkyl halide
Reagents: Strong base (OH⁻, OEt⁻, t-BuOK)
Product: Alkene
Name: Dehydrohalegenation
Start: Alkyl halide
Product: Alkene
Reagents: Strong base (OH⁻, OEt⁻, t-BuOK)
Name: Dehydrohalegenation
NaOH/t-BuOK
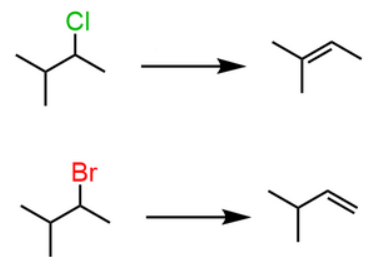
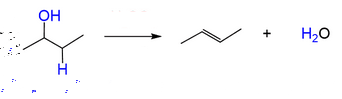
Start: Alcohol
Reagents: H₂SO₄ + heat
Product: Alkene
Name: Dehydration
Start: Alcohol
Product: Alkene
Reagents: H₂SO₄ + heat
Name: Dehydration
H₂SO₄ + heat
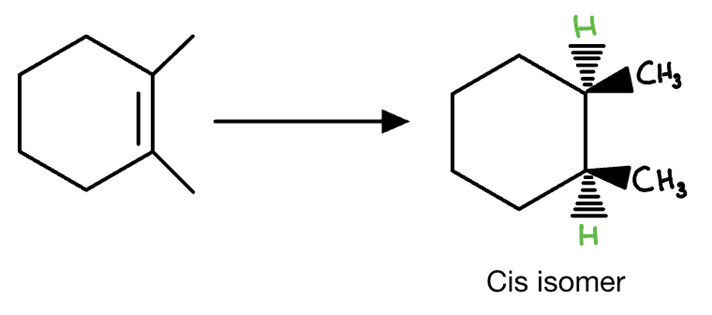
Start: Alkyne
Reagents: H₂, Lindlar
Product: Cis-alkene
Name: Cis Reduction
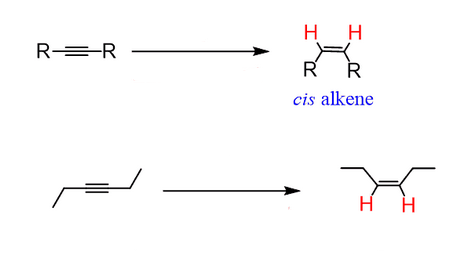
Start: Alkyne
Product: Cis-alkene
Reagents: H₂, Lindlar
Name: Cis Reduction
H₂, Lindlar
Start: Alkyne
Reagents: Na/NH₃
Product: Trans-alkene
Name: Trans Reduction
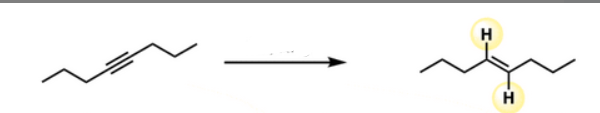
Start: Alkyne
Product: Trans-alkene
Reagents: Na/NH₃
Name: Trans Reduction
Na/NH₃
Start: Alkyne
Reagents: H₂, Pd/Pt/Ni
Product: Alkane
Name: Complete Reduction
Start: Alkyne
Product: Alkane
Reagents: H₂, Pd/C
Name: Complete Reduction
H₂, Pd/Pt/Ni

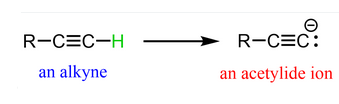
Start: Terminal alkyne
Reagent: NaNH₂
Product: Acetylide ion
Name: Deprotonation
Start: Terminal alkyne
Product: Acetylide ion
Reagent: NaNH₂
Name: Deprotonation
NaNH₂