2.6 Properties of Water
1/16
Earn XP
Description and Tags
A series of flashcards created to reinforce key concepts related to the chemical properties of water and its significance in biological systems.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
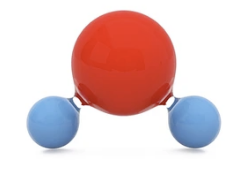
What is the chemical formula for water?
H2O
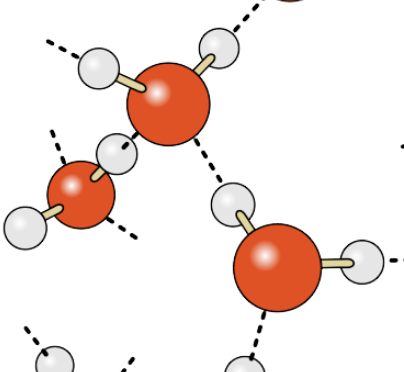
What type of bond occurs between within one water molecule? Often drawn as a solid line.
Covalent bond
What is a molecule called when its electrons are shared equally?
Nonpolar
What is a molecule called when its electrons are shared unequally?
Polar
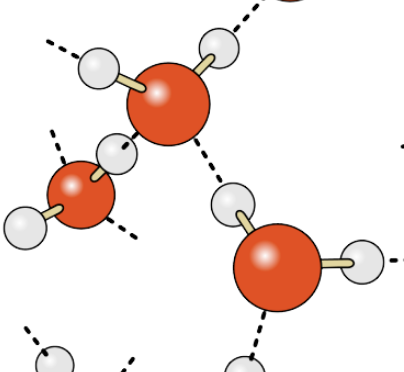
Which description is most accurate about water molecules? Water molecules are ___ because ___.
polar … electrons are shared unequally
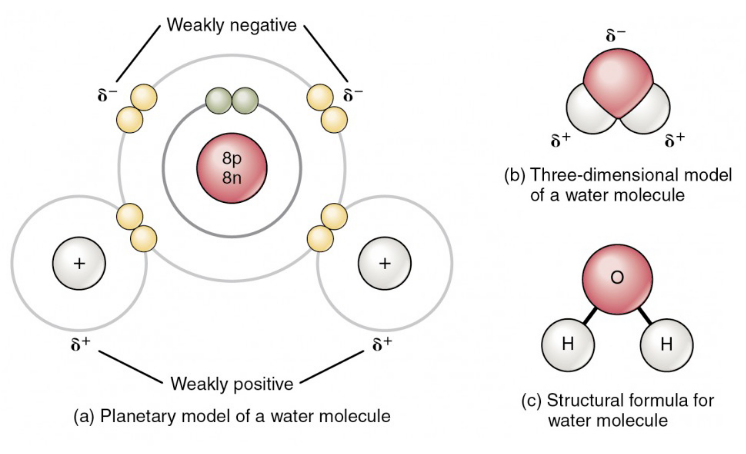
Which description about water molecules is most accurate?
The positively charged hydrogen of one water molecules is attracted to the negatively charged oxygen of another.
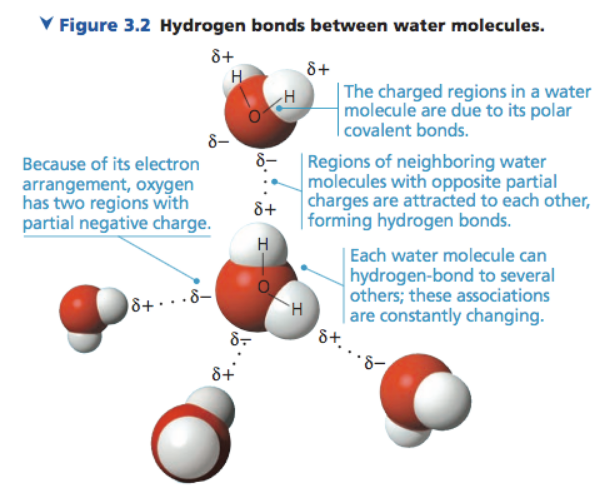
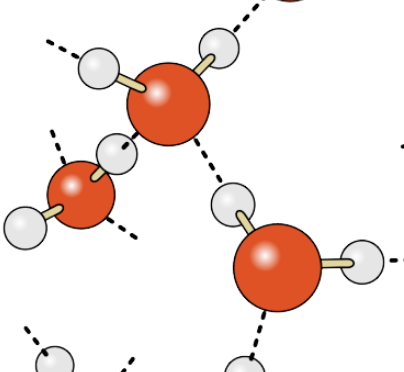
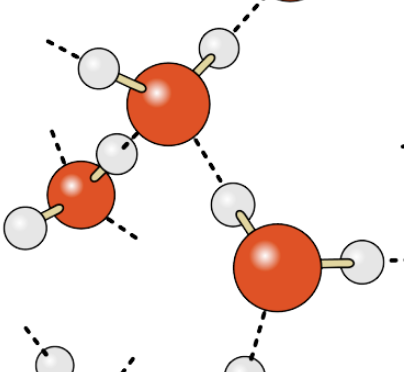
What type of bond occurs between different molecules of water? Often represented by a dashed line.
Hydrogen bond
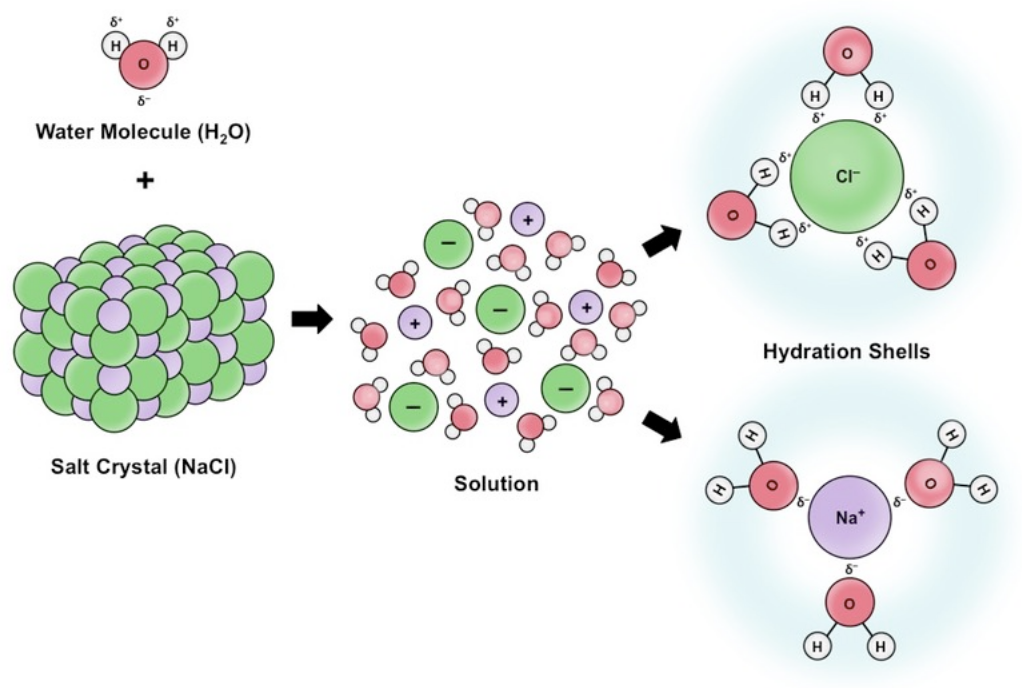
Why is water the most common solvent for living systems?
It dissolves many substances due to its polar nature, allowing for chemical reactions and essential biological processes.
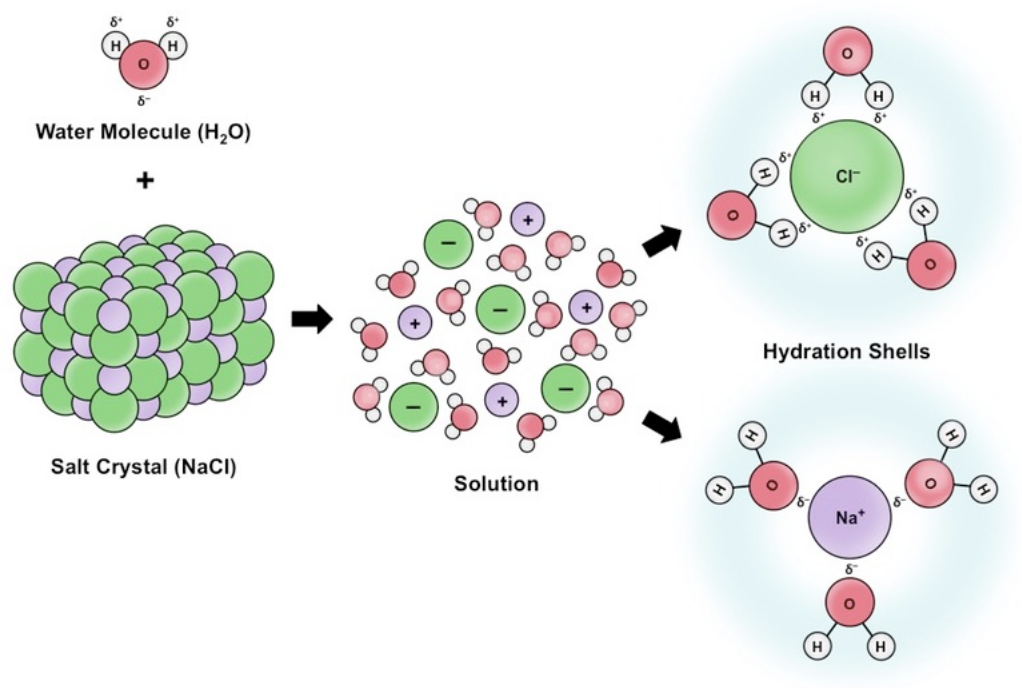
Which type of substances does water readily dissolve?
Polar
What is cohesion?
When molecules form hydrogen bonds with other molecules of the same type.
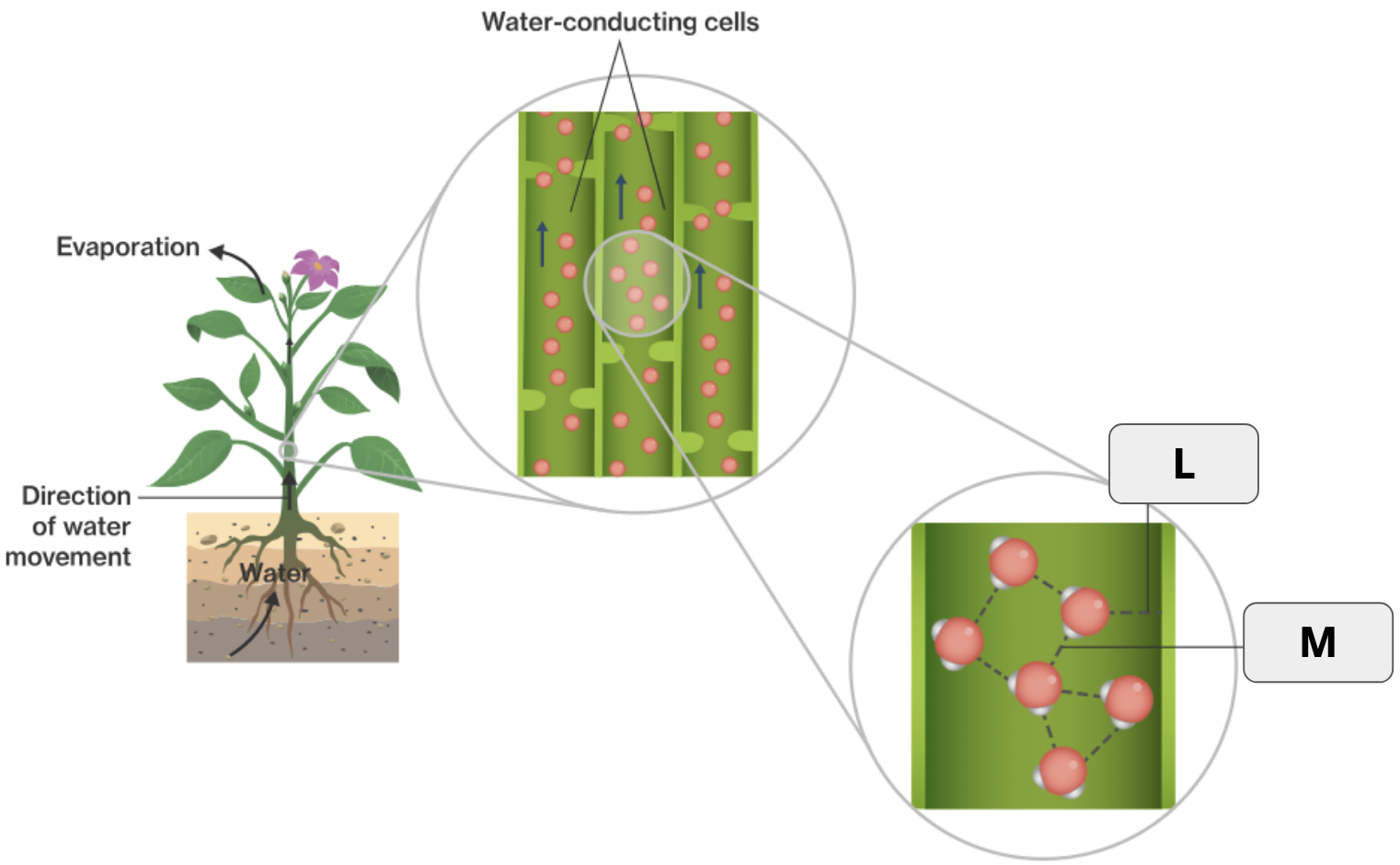
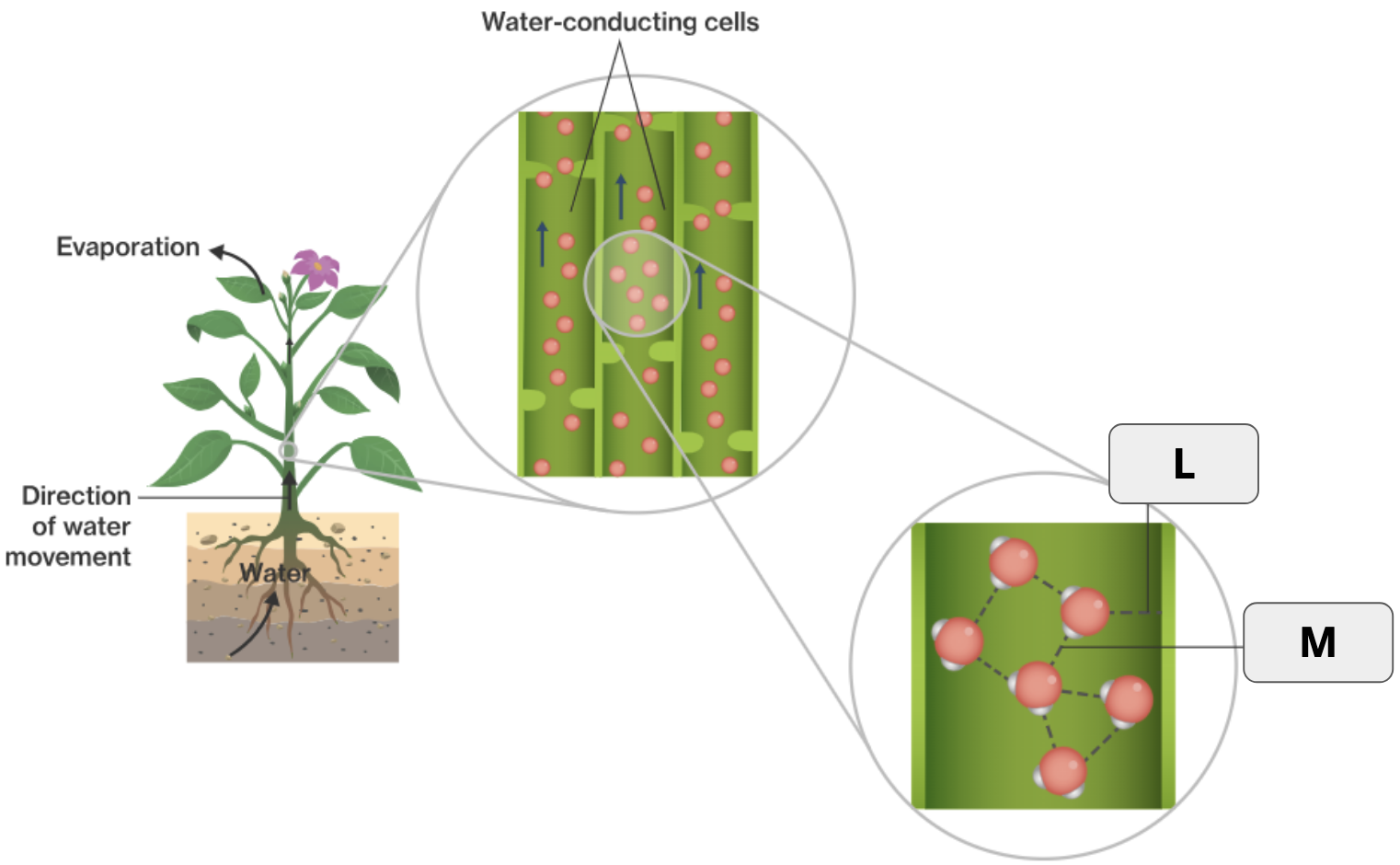
What is adhesion?
When molecules form hydrogen bonds with other molecules of a different kind.
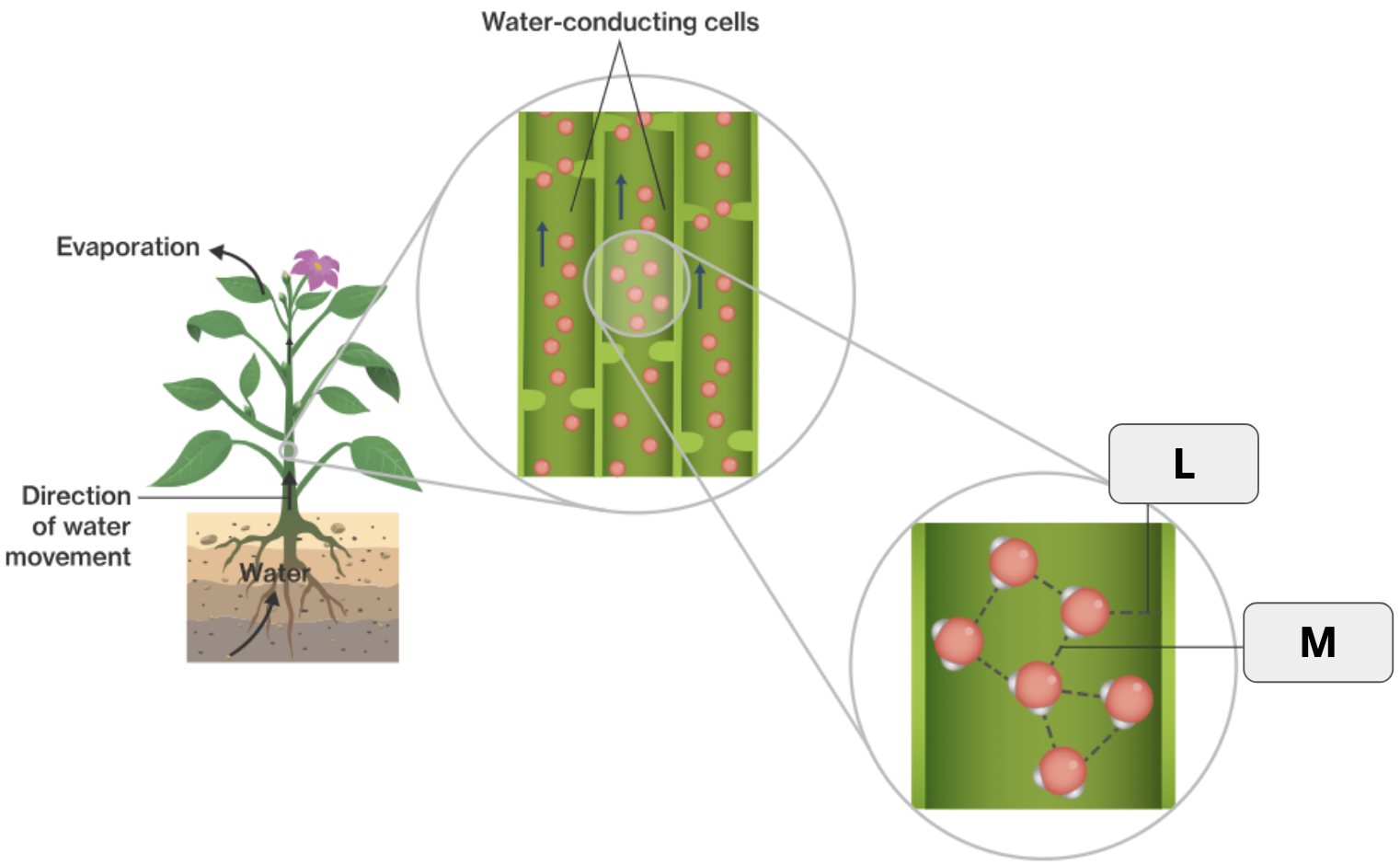
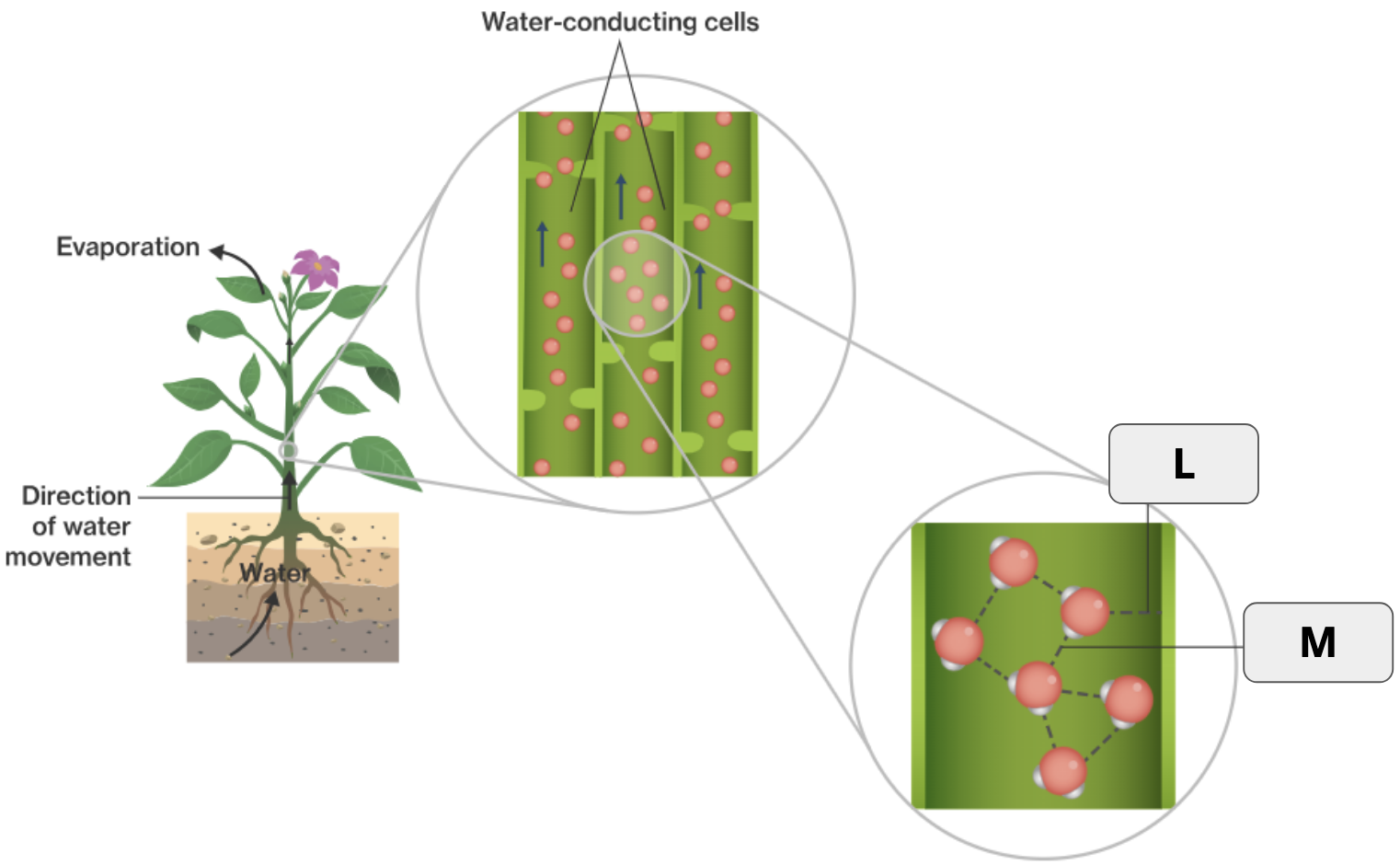
What is the specific term that refers to water being released as a gas from plants?
Transpiration
What is capillary action?
The process of liquid flowing in narrow spaces against gravity.
What is surface tension?
The phenomenon where a liquid's surface acts like a thin film due to attractive forces between its molecules.
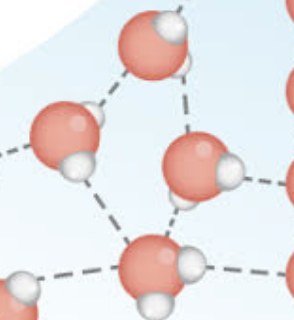
What can be said about the strength of hydrogen bonds compared to covalent bonds?
Hydrogen bonds are weak attractions, while covalent bonds are strong bonds.
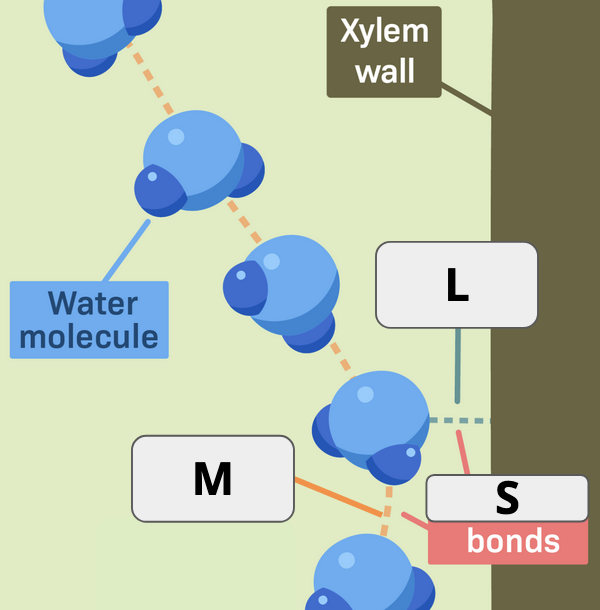
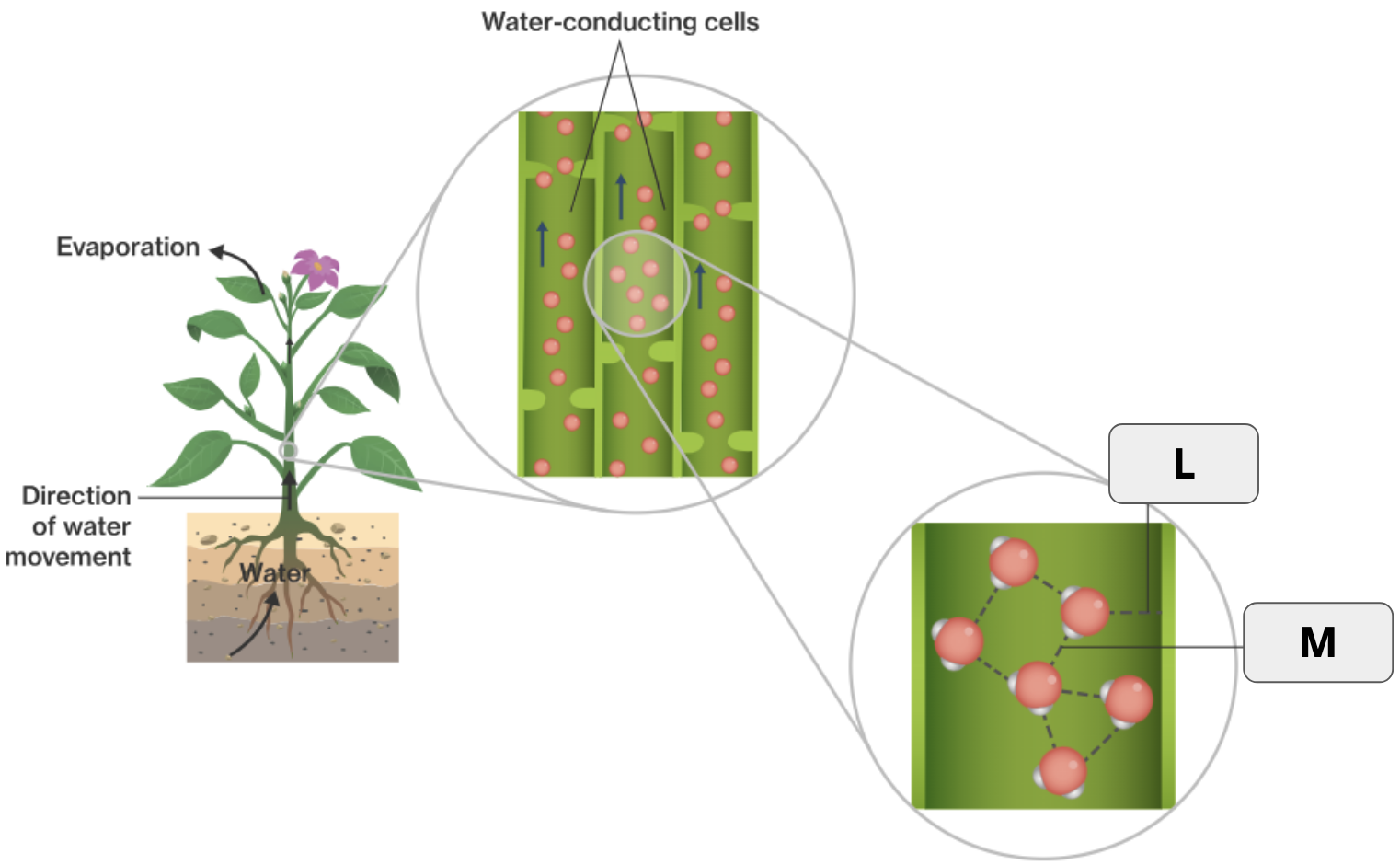
What two terms apply to “L”?
Hydrogen bond and Adhesion
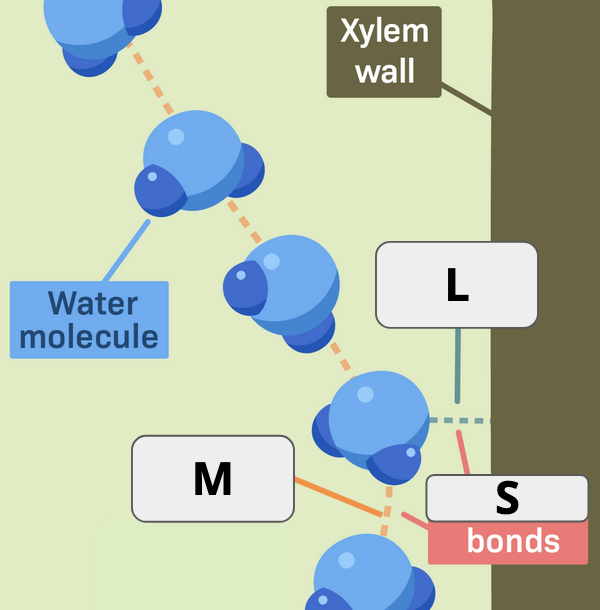
What two terms apply to “M”
Hydrogen bond and Cohesion