Genetic Diversity & Complex Traits
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
paralogous sequence meaning
Genes that come from the same ancestral gene but a slight change happened during duplicated that may make them differ from the original & each other.
E.g. α-globin and β-globin
CNVs (Copy Number Variation) are linked to what 2 problems
Link to dosage sensitivity and neurodevelopmental disorders
Haploinsufficiency may arise due to……… (2)
Heterozygous with one functional and one non-functional (null) allele
OR hemizygous (del) - allele deleted on one Chr
Example of haploinsufficient CNV microdeletion syndrome
Smith Magennis syndrome (SMS) – Haploinsufficiency of RAI1 gene (microdeletion)
Give an example of a single gene (mendelian) disease
Huntington’s disease
Give an example of a polygenic disease
Schizophrenia
Give an example where genes don’t play as much of a problem - it is based on environment
Tuberculosis
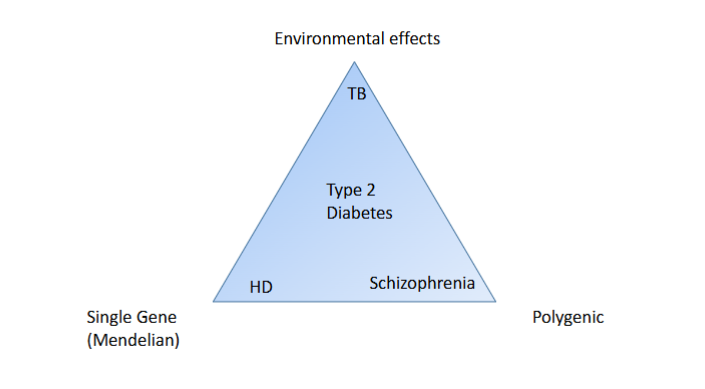
Are most diseases polygenic/mendelian?
Polygenic
True/False: Genes are always Mendelian
True
True/False: Characters (phenotype) are always Mendelian
False: Characters (phenotype) are never entirely Mendelian
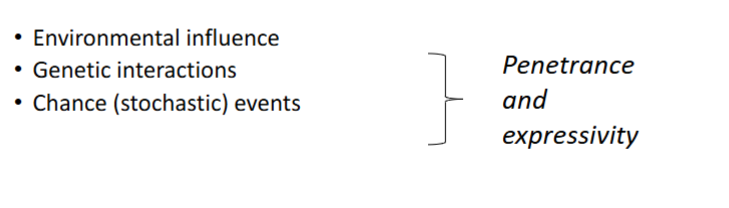
What are Multifactorial traits/disorders
Traits/disorders showing familial clustering, but no recognised Mendelian inheritance pattern
What determines the presentation of Multifactorial traits/disorders
Determined by the additive effects of many genes at different loci (polygenic), combined with effects of environmental factors
Give some examples of Multifactorial traits/disorders
Height , Type II Diabetes Mellitus, Hypertension, Cardiovascular disease, Schizophrenia, Alzheimer's disease
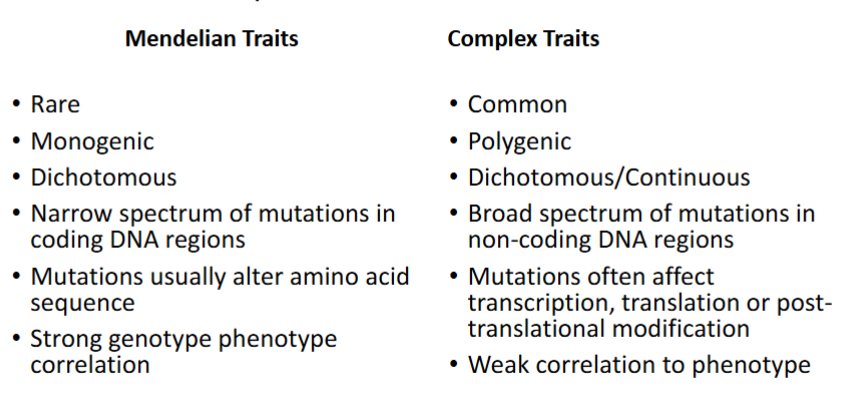
Mendelian Traits Vs Complex Traits (Know this!)
Common vs Rare
Mono/Polygenic
Dichotomous vs Continuous
Narrow / Broad spectrum of mutations
Associated with coding / non-coding DNA regions
Mutations alter what
Weak / Strong correlations to phenotype
What is the Polygenic Theory
A useful framework for considering the inheritance patterns of traits/disorders that rely on the interaction of a large number of genetic factors, each of which makes a small contribution to the overall phenotype
What are the 2 main concepts involved in teh polygenic theory
Heritability – estimates how much of the differences between populations are down to their genes
Thresholds – explain how dichotomous characters can be polygenic
Heritability
The proportion of the total phenotypic variance that is attributable to genetic variance in a population
When studying heritability is it measuring individuals / populations
Heritability is not about individuals – it relates to populations
(Compare incidence in relatives of affected individual vs incidence in general population)
True/False: Heritability of 0.5 = trait is 50% caused by genetic factors
False: Heritability of 0.5 does NOT mean that a trait is 50% caused by genetic factors, it means that 50% of the variability in the trait in a population is due to genetic differences among people
(e.g. religion has heritability of 0. Intelligence somewhere in between 0 and 1)
An example of heritability is PKU. What causes it
Caused by mutations in the PAH gene – phenylalanine hydroxylase
Mutations in PKU leads to what
Mutations prevent conversion of the aa phenylalanine to other compounds
Builds up to toxic levels, affecting nerve cells – brain damage
What is the heritability difference (proportion of the total phenotypic variance that is attributable to genetic variance in a population) of PKU in Ireland in 1920 (before testing for PKU & 2020?
There was a higher heritability in 1920 than in 2020 as people in 1920 weren’t diagnosed, therefore didn’t change their diet to accommodate for the disease and they would all manifest the phenotype. In 2020, less people developed the phenotype as they changed their diet to prevent the manifestation of the disease.
Twin studies are very useful for acquiring Evidence for Genetic involvement in Complex Diseases. What are some other studies that are useful for this?
• Family Studies: Weaker evidence as family environment is shared and so could be responsible for the effect
• Adoption studies: Stronger evidence than family studies as separates the effects of genes and family environment
• Adopted twin studies: Very rarely happens, but very strong evidence as genetics are matched and family environment is different
What theory explains how dichotomous characters can be polygenic
Threshold Theory
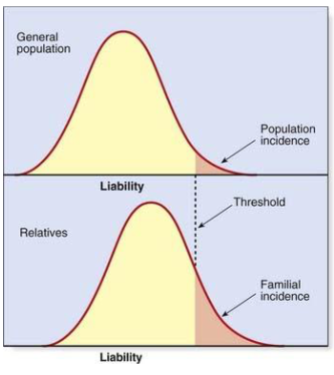
What is the Threshold Theory
Susceptibility to a disease is a continuous character that depends on combined effect of many genes.
If your susceptibility exceeds a threshold you will manifest the disease – “all or nothing
True/False: Threshold theory states that relatives will be more likely to manifest the disease than general population
True
(Explains why complex diseases tend to run in families)
John and Mary have a son with a cleft palate (polygenic) and two normal children.
Mary’s sister Julie has two children with a cleft palate and a normal child.
Which sister is more likely to have another child with a cleft palate?
Julie
(Cleft palate is polygenic. Polygenic threshold characters run in families. Parents with several affected children have more high risk alleles than parents with one affected)
Recurrence risk rules for Multifactorial Inheritance
• The more seriously affected, the higher the risk for sibs
• The more affected children you have, the higher risk of recurrence
• The closer the relative is to the index case, the higher the risk of recurrence
Give an example of a Gender Biased Polygenic Inheritance
Hypertrophic Pyloric Stenosis
Hypertrophic Pyloric Stenosis symptoms
Projectile vomiting, failure to thrive
Explain the Gender Biased Polygenic Inheritance associated with Hypertrophic Pyloric Stenosis symptoms
5 times more common in males
Must be higher threshold for girls than boys
Who is more likely to manifest Hypertrophic Pyloric Stenosis?
• Offspring of affected males
• Offspring of affected females
Offspring of affected females
The Carter effect: higher recurrence risk if the index case is of the less commonly affected sex. Since females require more risky genes for this to occur (higher threshold) then its much more likely she will pass on some of those genes.
How do we find Genes Involved in Complex Disorders? What tests?
• Linkage analyses
• Association
• Candidate gene testing
• Genome-wide association studies (GWAS)
What is Linkage
Linkage is a relationship between loci (not alleles). It is a specifically genetic phenomenon
Linkage analysis looks at what
Looks at physical chunks of the genome of related individuals with the phenotype and associates them with given traits
What is the principle behind Linkage analysis
If we find a common genetic marker (e.g. microsatellite, SNP), we assume that the gene that causes the disease is somewhere in the same area.

What is Genetic Association
Goal?
It is a purely statistical phenomenon and not specifically genetic.
Goal: identify one or more alleles within a population that co-occur with a particular phenotypic trait more often than would be expected by chance
What is the method used to test Genetic Association
gather some people with a disease (cases) and some people without a disease (controls) in a population and look to see what alleles are present more in cases than controls
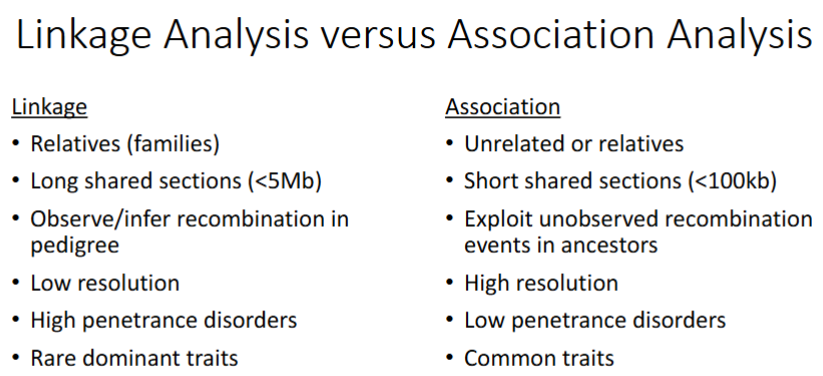
Linkage Analysis vs. Association Analysis
Studies Unrelated People /
Long/Short Relatives shared sections (kb)
Goal
High/Low resolution
High/Low penetrance disorders associated
Common/Rare traits
Candidate gene testing is a genetic testing approach that focuses on specific genes thought to be involved in a disease, rather than scanning the entire genome.
How are target genes for candidate gene testing selected
Targets are chosen based on existing knowledge of the disease or by genetic linkage/association studies
Positional Cloning combines what 2testing strategies
Linkage & Association Strategy
Positional Cloning method
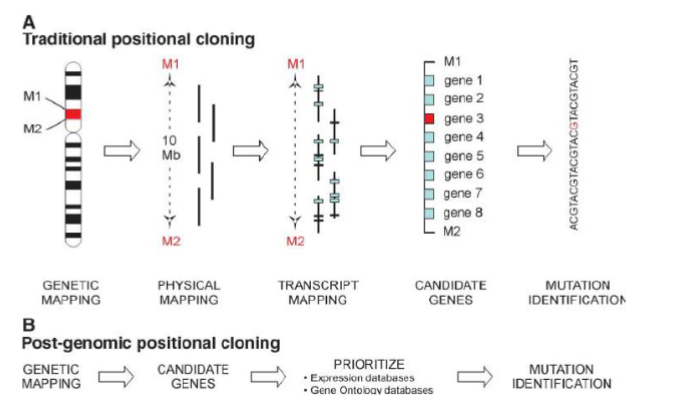
Genome Wide Association Studies tests what
Typically test hundreds or thousands of individuals.
Scans all genes in the genome with no prior knowledge needed to find links and associations between traits and variants
What is Genome-wide polygenic scores
Possibly the future of clinical genetics.
Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations (know what everyone is at risk of from their genes to manage their lifestyle to prevent the manifestation of these disorders)
Compare Type 1 & 2 diabetes
Type 1 sudden onset in youth related to autoimmune pancreatitis
Type 2 gradual onset in middle/later years associated with obesity and inactivity (diabetes epidemic)
What is MODY
Maturity onset diabetes of the young
MODY pattern of inheritance
Autosomal dominant pattern of inheritance
(7 different single gene defects identified)
Is MODY associated with obesity & sedentary lifestyle
No
What are some stats that provide evidence for genetic factors in type 2 diabetes
Ethnic differences in prevalence
Family and twin studies – 2.4 fold risk for families
15-25% of first degree relatives develop impaired glucose tolerance or T2D
More than 30 genes with susceptibility alleles (linkage/GWAS studies)
Mitochondrial Type 2 Diabetes is associated with several single gene defects, some of which are associated with what problem
Deafness
Is behaviour associated with genetics
Yes all of these are:
Intelligence
Assertiveness
Aggressiveness
Submissiveness
Self discipline
Hard working
Lazy
Sexual Orientation
Interconnects with value and judgments