Biological Chemistry: Atoms, Bonding, and Intermolecular Forces
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
What are the building-block elements of biological molecules?
Carbon (C), Hydrogen (H), Nitrogen (N), and Oxygen (O)
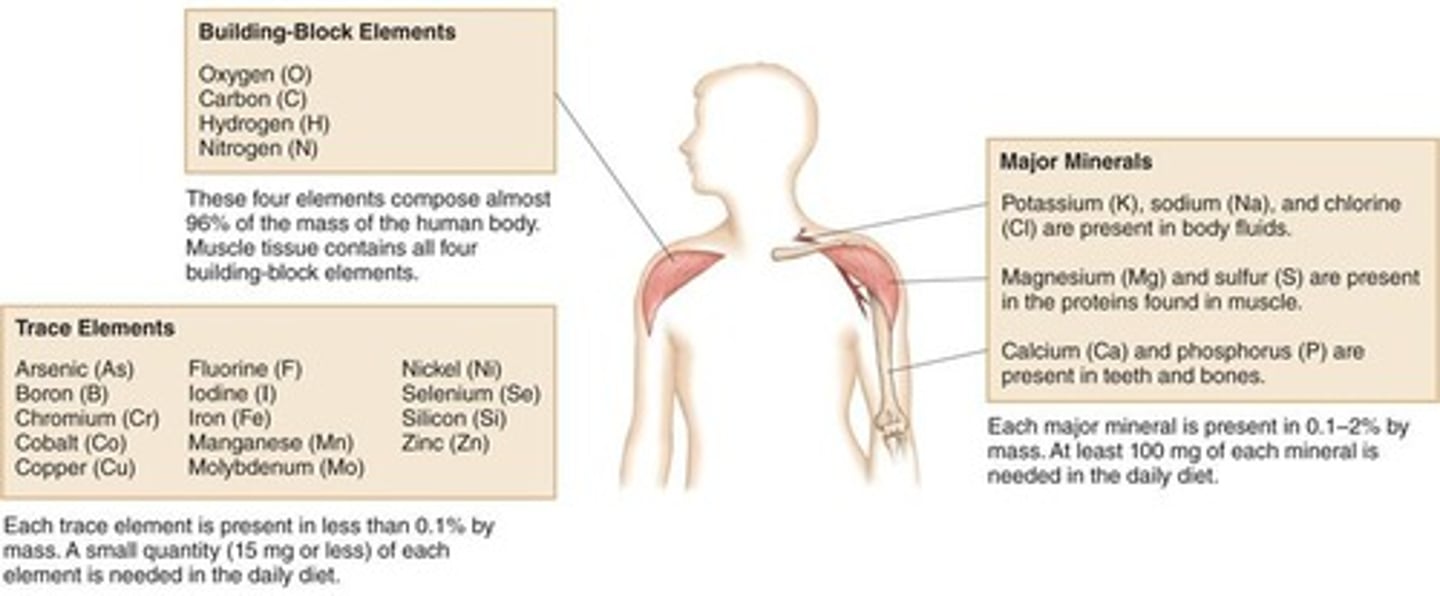
What percentage of the human body is made up of the elements C, H, N, and O?
96%
Name two major minerals essential for biological functions.
Sodium (Na) and Potassium (K)
What are trace elements, and why are they important?
Trace elements like Iron (Fe), Copper (Cu), and Zinc (Zn) play important roles in protein molecules.
What is an ionic bond?
An electrostatic attraction between oppositely charged ions.
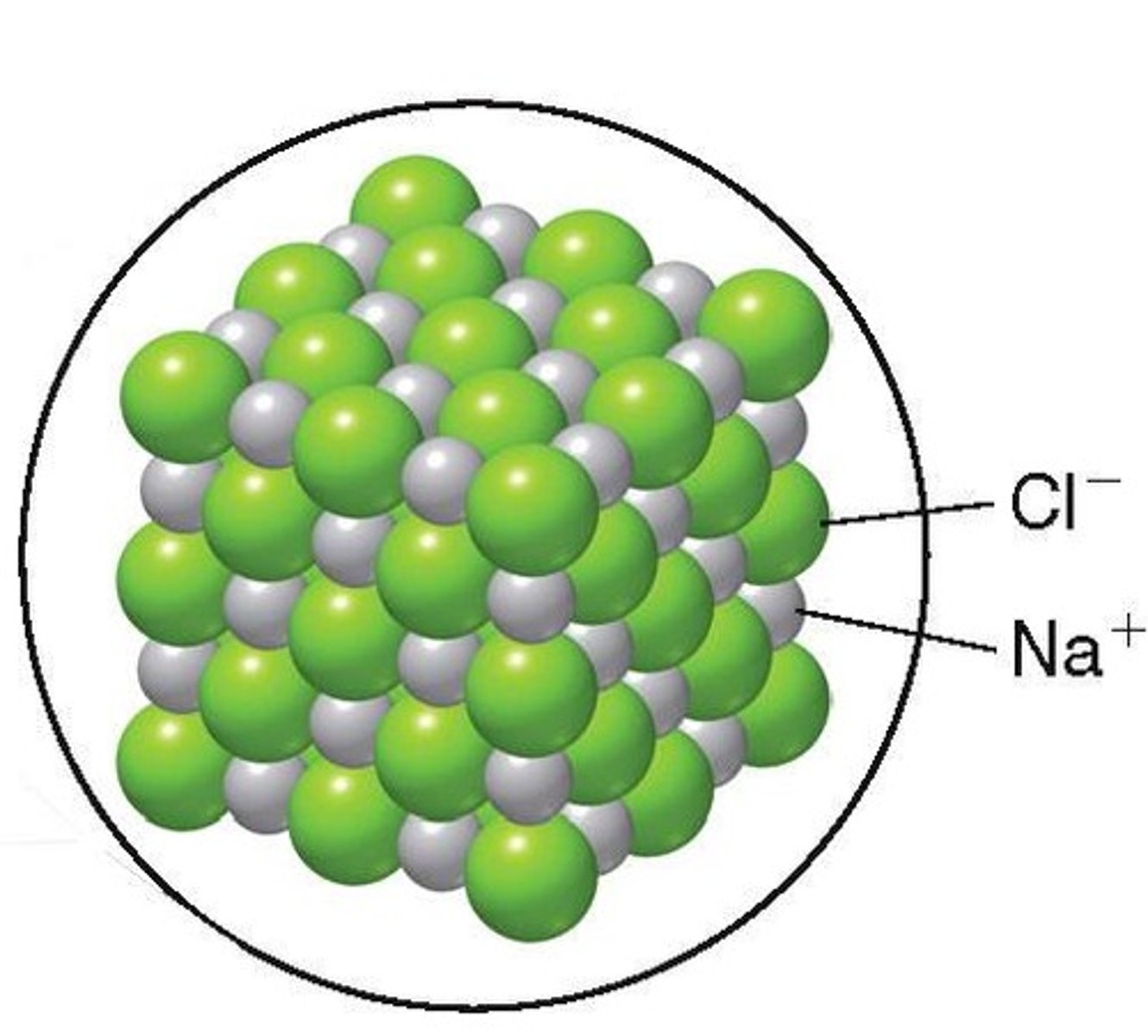
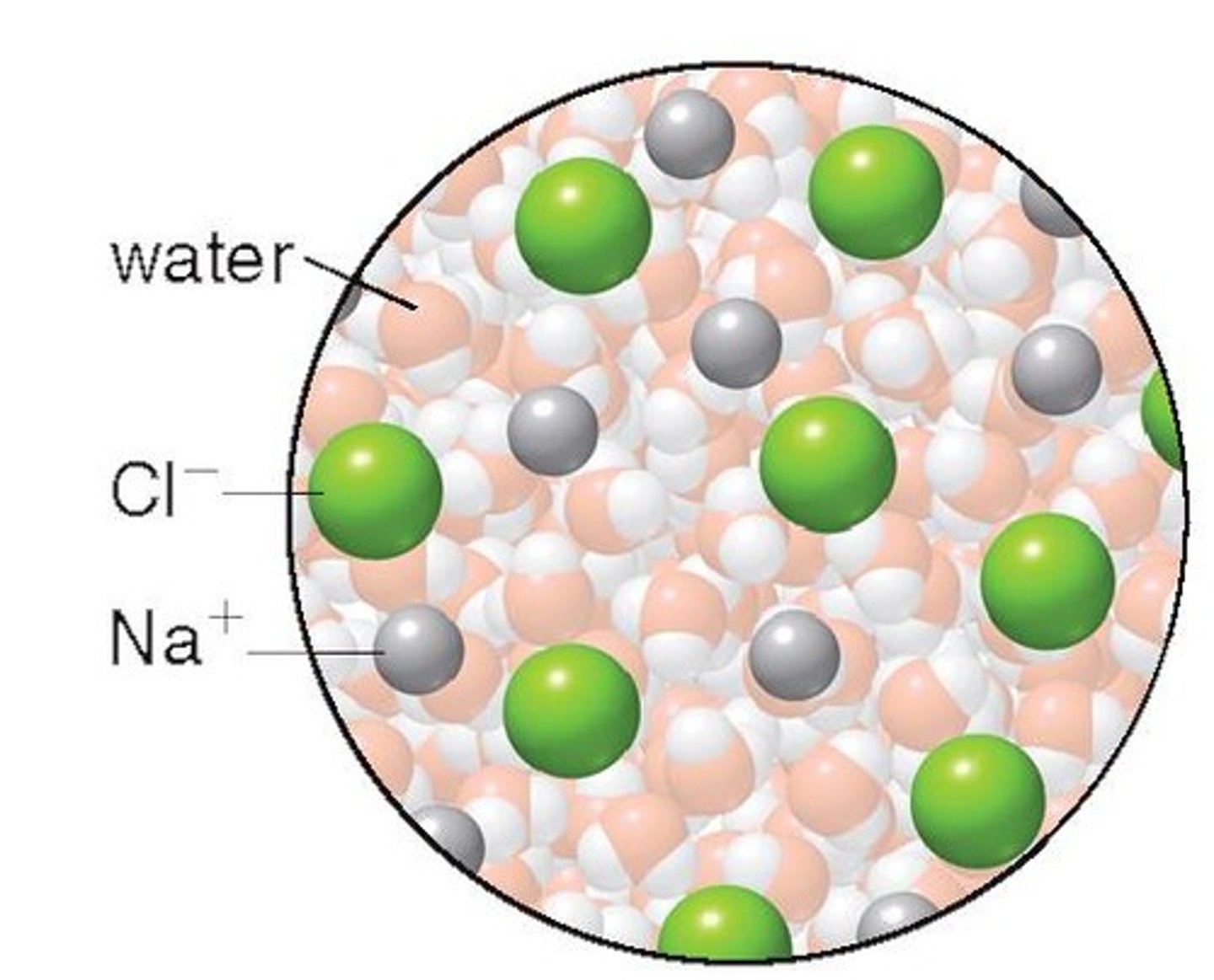
Why are ionic bonds not useful for building biological molecules?
They do not have specific shapes and can break in water.
What is a covalent bond?
A bond formed by sharing electrons.
How do covalent compounds behave in water?
Covalent bonds usually do not break by dissolving in water.
What are intermolecular forces?
Forces that occur between molecules, including London dispersion forces, dipole-dipole interactions, and hydrogen bonds.
What are hydrogen bonds?
Electrostatic attractions between a δ+ hydrogen atom and a δ- atom (N, O, or F) in another molecule.
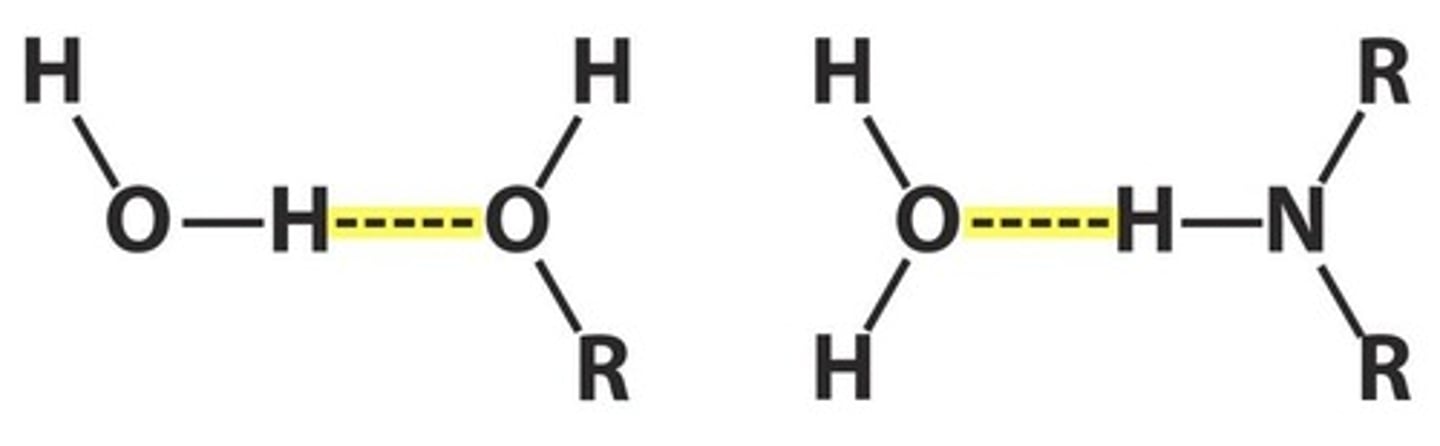
How do hydrogen bonds compare to dipole-dipole interactions?
Hydrogen bonds are stronger than typical dipole-dipole interactions but weaker than ionic bonds.
What is the role of hydrogen bond donors and acceptors?
Each hydrogen bond has a donor (the molecule with the hydrogen) and an acceptor (the electronegative atom).
What are the four types of intermolecular forces?
London dispersion forces, dipole-dipole interactions, hydrogen bonds, and electrostatic attraction.
What is the significance of intermolecular forces in biomolecules?
They play important roles in determining biomolecule structure and interactions.
What is the electronegativity trend for H, C, N, O, and F?
Electronegativity increases from H/C to N/O/F.
What is the typical bond angle in covalent compounds?
Covalent compounds have specific geometries that determine bond angles.
What happens to ionic bonds in water?
Ionic bonds tend to break in water, losing their structural integrity.
What is the primary function of biological molecules?
To transform energy and carry information necessary for life.
What is the relationship between structure and function in biological molecules?
Function follows structure; the shape of a molecule determines its function.