Titration calculations 1
1/3
Earn XP
Description and Tags
https://www.youtube.com/watch?v=x8DLLCNMKAs&list=PL9IouNCPbCxXDlRtCQEG0cGehBvJ7t9Pf&index=12
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
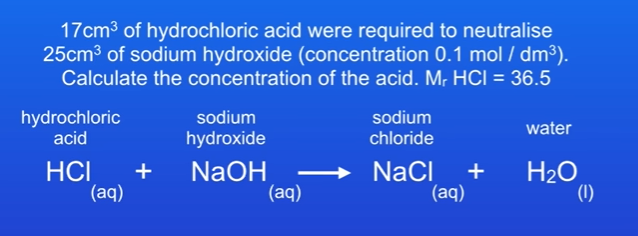
17cm³ of hydrochloric acid were required to neutralise 25cm³ of sodium hydroxide ( concentration 0.1 mol / dm³) Calculate the concentration of the acid.
Write c=, n= and v= under the acid and alkali.
Enter the info that has been given, and convert units do dm³ by dividing by 1000.
Concentration of NaOH is 0.1 mol / dm³ and volume is 0.025dm³ (25/1000).
Volume of hydrochloric acid is 0.017 dm³
Calculate number of moles of NaOH . (concentration x volume)
Number of moles = 0.1 × 0.025. = 0.0025 moles.
No big numbers infront of any molecules, therefore we must have same amount of moles for hydrochloric acid, which is 0.0025 moles as well.
We can now calculate concentration of HCl by doing number of moles / volume. 0.0025 / 0.017. Which is 0.147 mol / dm³ to 3d.p.
How to work out number of moles
Concentration x volume.
How do we calculate concentration of acid in grams / dm³
By doing concentration in mol / dm³ x Mr.
How to work out concentration
Concentration = moles / Volume.