Gram-negative rods (non-Enterobacteriaceae)
1/77
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
78 Terms
GI
GI or respiratory pathogen? Campylobacter jejuni
GI
GI or respiratory pathogen? Helicobacter pylori
respiratory
GI or respiratory pathogen? Haemophilus influenzae
GI
GI or respiratory pathogen? Vibrio species
Respiratory
GI or respiratory pathogen? Legionella pneumophila
respiratory
GI or respiratory pathogen? Bordetella pertussis
Curved rods, motile with polar flagella, oxidase +, infects GI epithelium, diagnosed with stool culture
Exception: Vibrio vulnificus invades bloodstream and is diagnosed with blood culture
Common themes of gram negative rods excluding Enterobacteriaceae: shape, motility, oxidase, infection site, diagnosis
Also comment on the exception
it is hard to culture
Important note in trying to diagnose H. pylori
aquatic, many are halophiles, alkali-tolerant
Preferred conditions of Vibrio species
Cholera, a severe diarrheal disease that kills by hypovolemic shock
endemic in developing nations
Vibrio cholerae causes —
a large inoculum is required to cause disease (108 cfu)
V. cholerae is acid-labile, therefore —
Abrupt onset diarrhea and vomiting
Non-inflammatory, so no WBC in stool
Fluid loss of 20-30 L/day
“Rice-water” stool
Lethargy
acidosis with Kussmaul breathing
Poor skin turgor
Presentation of cholera
Mucinase (helps V. cholerae penetrate intestinal mucosal layer to adhere to epithelial cell)
Flagellum (motility aids movement through mucosal layer)
Toxin co-regulated pilus (surface structure that allows attachment to epithelium)
Cholera toxin (most important, causes ADP-ribosylation of Gs, so adenylate cyclase continually active, cAMP production leads to massive ion secretion)
4 virulence factors of Vibrio cholerae
Culture from stool in Thiosulfate-Citrate-Bile Salts-Sucrose high pH medium –
high pH inhibits enterobacteriaceae,
sucrose in plate is fermented to produce yellow colonies
Diagnosis of cholera
Rehydration/electrolyte replacement is most critical
Antibiotics and reduce transmission and shorten infection duration, but resistance is increasing
Prevention: VaxChlora used for travelers to endemic areas
Therapy for cholera
Contaminated seafood or water (oysters, shellfish from warm water)
Vibrio parahaemolyticus: transmission
forms pores in cell membranes that lead to ion disruption
Vibrio parahaemolyticus: how it causes disease
Explosive, watery diarrhea and vomiting, fever
(typically self-limiting disease)
Vibrio parahaemolyticus: symptoms
B-hemolytic
Vibrio parahaemolyticus: hemolysis
Grows on TCBS agar; PCR
Diagnosis of Vibrio parahaemolyticus:
Volume replacement, supportive care in mild cases, IV antibiotics in cases of septicemia
Therapy for Vibrio parahaemolyticus:
liver disease, hemochromatosis
Severe outcomes of Vibrio parahaemolyticus infection are more likely in patients with — or —
Occurs after ingesting contaiminated seafood
Crosses the intestinal wall, enters the bloodstream, spreads systemically
Vibrio vulnificus: pathogenesis
gastroenteritis, then sepsis, then bullous skin lesions
flaccid, hemorrhagic bullae
hypotensive shock, disseminated intravascular coagulation, multi-organ failure
50% mortality rate even with treatment
Vibrio vulnificus: symptoms
Necrotizing fasciitis, bullous skin lesions, sepsis and systemic shock
Rapid onset, symptoms appear within hours of exposure
can require amputation
Vibrio vulnificus: presentation of cases in which contaminated seawater enters wounds
phase variable polysaccharide capsule
MARTX (Multifunctional Autoprocessing RTX Toxin), which disrupts host cytoskeleton and facilitates tissue destruction and necrosis
Iron acquisition systems (strong Fe requirement; increased risk in patients with iron overload)
Vibrio vulnificus: 3 virulence factors
clinical presentation and history: severe wound infection or septicemia after seafood ingestion/seawater exposure, rapidly progressing bullous skin lesions
Culture from wounds, blood, or stool and grow on Thiosulfate-Citrate-Bile Salts-Sucrose (TCBS) Agar: green colonies produced, PCR for rapid detection
Vibrio vulnificus: diagnosis
IV antibiotics (doxycicline + 3rd gen cephalosporin)
Wound debridement/amputation in cases of wound infection
ICU management of septic shock, hypotension, organ failure
Vibrio vulnificus: treatment
Gram negative, curved or S-shaped, motile with multiple polar flagella
Helicobacter pylori: Gram, shape, motility
microaerophilic (requires low oxygen levels to survive)
Helicobacter pylori: oxygen requirement
positive for all
Helicobacter pylori: catalase, oxidase, urease
stomach epithelium, especially antrum
Helicobacter pylori: primary colonization site
Production of urease converts urea to ammonia, neutralizing stomach acid. Ammonia and bacterial toxins damage gastric mucosa.
How Helicobacter pylori leads to ulcers
Chronic inflammation caused by Helicobacter pylori increases cancer risk, it also triggers excessive B-cell activation, which can lead to Gastric Mucosa-Associated Lymphoid Tissue B-cell Lymphoma (MALT lymphoma)
Helicobacter pylori and cancer
family clustering observed (monozygotic twins show higher infection concordance than dizygotic twins)
Helicobacter pylori: genetic susceptibility
Flagella—able to swim through this gastric mucosal layer
Mucinase & proteases—degrade gastric mucous
Adhesins—uses SabA and BabA to bind gastric epithelium
Cytotoxins—vacuolating cytotoxin & cytotoxin-associated gene A (disrupts cell signaling)
Urease—raises pH by urea—>ammonia
Helicobacter pylori: 5 virulence factors
endoscopy; This is typically done when the patient has symptoms severe enough to warrant endoscopy or if there is suspicion of a more serious condition (e.g., gastric cancer).
Tests like histology and rapid urease test can be performed on the collected tissue sample
Diagnosis of H. pylori: invasive test
urea breath test: The patient ingests a urea solution labeled with a carbon isotope. If H. pylori is present, it breaks down the urea, releasing carbon dioxide that can be detected in the patient's breath.
Stool antigen assay: Detects H. pylori antigens in a stool sample. It's a noninvasive method that's effective for diagnosing active infection.
Patient needs to off of abx and proton pump inhibitors before these tests are conducted.
Diagnosis of H. pylori: non-invasive test
Proton Pump Inhibitor (PPI): Suppresses gastric acid production, allowing healing of ulcers and creating a less acidic environment for the antibiotics.
Antibiotics (2 drugs):
Common choices include clarithromycin and amoxicillin or metronidazole (based on local resistance patterns).
Bismuth Subsalicylate: This has a protective effect on the stomach lining and has mild antibacterial activity against H. pylori.
Treatment of H. pylori
After completing treatment, it's important to confirm eradication of the infection. This can be done using noninvasive tests like the urea breath test or stool antigen test, usually 4 weeks after completion of therapy.
Treatment failures are common and can be due to abx resistance, treatment non-adherence, or reinfection.
Post treatment of H. pylori:
microaerophilic, catalase +, grows at 42 degrees C (body temp of birds), curved or S shaped
Campylobacter jejuni: oxygen needs, catalase, temperature, shape
Campylobacter jejuni is most commonly transmitted through the fecal-oral route, primarily by consuming contaminated food, especially undercooked poultry. It can also be transmitted through unpasteurized milk or contaminated water.
Infectious Dose (ID50): The infective dose is relatively low, around 10,000 organisms, meaning that ingesting a small amount of contaminated food can lead to infection.
Campylobacter jejuni: transmission and infectious dose
Campylobacter jejuni is invasive and can penetrate the epithelial lining of the small intestine, causing inflammatory gastroenteritis.
Campylobacter jejuni: what does it infect?
The early phase involves fever, chills, headache, and myalgia (muscle pain). This is followed by gastrointestinal symptoms.
Gastrointestinal Symptoms:
Watery diarrhea (in most cases).
In about 15% of cases, diarrhea may become bloody.
Abdominal cramps are common, and nausea affects about 25% of patients.
The illness can range from mild to severe, with some cases progressing to sepsis.
Self-limiting Disease:
Most infections are self-limiting and resolve within 7 days, even without treatment. However, the severity can vary.
Campylobacter jejuni: clinical features
In patients with HIV/AIDS or other immunocompromised states, the infection can be prolonged and more severe.
0.1% of patients may develop Guillain-Barré Syndrome. The mechanism of GBS is thought to involve molecular mimicry between Campylobacter’s surface antigens and the human nervous system.
About 10% of people with Campylobacter infections experience joint pain, and around 2-3% may develop reactive arthritis, an autoimmune condition.
In some cases, Campylobacter jejuni infection can present as pseudoappendicitis, where the symptoms mimic appendicitis, but without the typical diarrhea.
Campylobacter jejuni: possible complications
Stool Culture: The gold standard for diagnosing Campylobacter jejuni infection is a stool culture, but it requires special conditions because the bacterium is microaerophilic.
PCR: Molecular methods such as PCR (polymerase chain reaction) are also used for quicker and more accurate identification.
Diagnosis of Campylobacter jejuni:
Supportive Care: Most cases of Campylobacter infection are self-limiting, and hydration and electrolyte replacement are the mainstays of treatment.
Antibiotics: Antibiotics are typically not needed in uncomplicated cases, but macrolides (such as azithromycin) may be used for severe cases or immunocompromised patients.
Prevention: Thorough cooking of poultry is critical to prevent infection. Practicing good hygiene and avoiding consumption of unpasteurized milk and untreated water also helps prevent infection.
Treatment of Campylobacter jejuni:
Flagella: motility helps to penetrate GI mucosa
Cytolethal Distending Toxin (CDT): disrupts host cell cycle and induces cell death.
Adhesins: allow it to adhere to and colonize the epithelial cells of the gastrointestinal tract.
Bacterial Antigens and Molecular Mimicry:
The antigens on the surface of Campylobacter jejuni are similar to gangliosides found on the surface of neurons—thought to be responsible for autoimmune responses like Guillain-Barré Syndrome (GBS).Virulence Plasmid:
Some strains of Campylobacter jejuni harbor a virulence plasmid that is associated with the ability to cause bloody diarrhea and higher disease severity.
Campylobacter jejuni: 5 virulence factors
Campylobacter jejuni is a major cause of bacterial foodborne gastroenteritis in the United States, with an estimated 2 million cases per year.
Campylobacter jejuni is primarily a zoonotic pathogen, meaning it can be transmitted from animals to humans.
Campylobacter jejuni: epidemiology
Gram negative, rods/coccobacilli, non-motile, facultative anaerobe
Haemophilus influenzae: Gram, shape, motility, oxygen needs
Capsule and Serotypes:
Encapsulated strains of Haemophilus influenzae are divided into six serotypes (a-f) based on the structure of their capsule.
The type b strain (Hib) was historically the most significant pathogen.
Nonencapsulated Strains:
Non-typeable Haemophilus influenzae (NTHi) do not cause as severe disease but are still significant pathogens.
Since the introduction of the Hib vaccine, NTHi strains have become the most common cause of Haemophilus influenzae infections.
Haemophilus influenzae: capsule vs nonencapsulated
upper respiratory tract
sinusitis, otitis media, bronchitis, pneumonia
Nonencapsulated H. influenzae commonly colonizes the — and can cause infections like —
Hib strains are not common colonizers of the upper respiratory tract but can cause more severe infections when they invade the body.
Severe Local Disease: Hib can lead to serious local infections, such as:
Epiglottitis, which can cause difficulty breathing and airway obstruction.
Pneumonia
Invasive Disease: Meningitis
Encapsulated H. influenzae colonizes where? Causes what diseases?
capsule—antiphagocytic
polyribitol phosphate (PRP)—helps the bacterium evade the immune system by preventing recognition and clearance by immune cells (is the part of the bacteria that’s the basis for the vaccine)
IgA1 proteases—cleaves IgA1 antibodies on mucosal surfaces
Lipopolysaccharide—endotoxin that triggers a serious inflammatory response
Adhesins, pili, fimbriae—used to bind epithelial cells in respiratory tract
H. influenzae virulence factors (4)
Haemophilus influenzae can commonly colonize the nasopharynx asymptomatically. Infection can occur under certain conditions (e.g., when immunity is compromised or other pathogens are involved).
Spread through respiratory droplets—once inhaled, they colonize oropharynx
Can translocate across epithelial/endothelial cells—once in the bloodstream, they spread and cause meningitis, epiglottitis, pneumonia, and sepsis.
H. influenzae transmission and colonization
1-3 days of mild URI followed by typical meningitis symptoms like fever, nuchal rigidity, severe headache, nausea, vomiting, irritability
Pathogenesis of H. influenzae meningitis
5-15% of survivors at risk for long-term neurological effects
•deafness, paresis, mental impairment
Complications of of H. influenzae meningitis
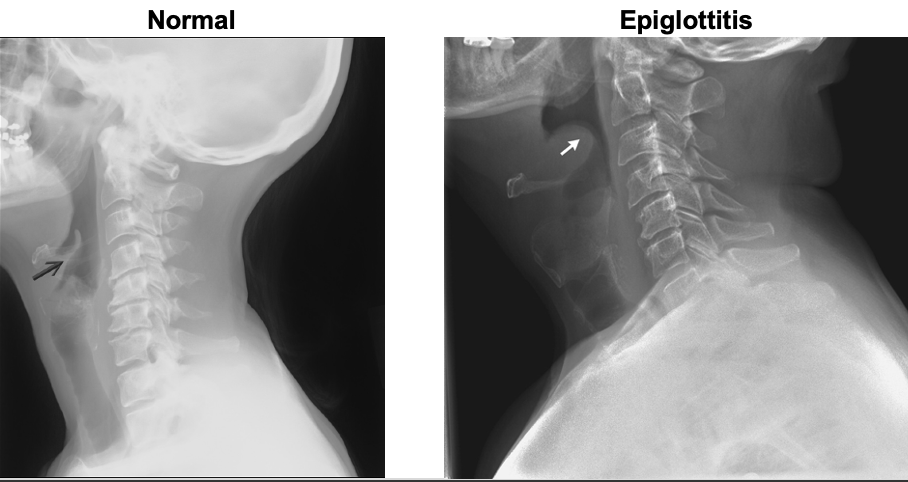
“thumb sign”
Epiglottitis appearance on X-ray
it often causes infections in individuals with weakened immune defenses, such as those with COPD or after a viral infection
Nontypeable Haemophilus influenzae is considered an opportunistic pathogen because
Culture and Gram stain: obtaining samples from relevant sites of infection; appears as small Gram-negative rods on Gram stain
Antigen detection: immunoassays can detect the PRP capsule component (limited utility in high vaccination countries
PCR: included in common respiratory PCR panels, highly sensitive and fast
Diagnosis of H. influenzae
β-lactam antibiotics (e.g., amoxicillin, ampicillin)
There is increasing antibiotic resistance in NTHi strains, which may require the use of cephalosporins, macrolides, or other broad-spectrum antibiotics
Rifampin can be used as post-exposure prophylaxis for close contacts (esp. unvaccinated)
Treatment of H. influenzae
Strict aerobe, Gram negative, nonmotile
Coccobacillus shape
Nutritionally demanding—difficult to grow outside the body, dies quickly in resp. secretions
Bordetella pertussis: oxygen requirements, Gram, motility, shape, nutrition requirements
does not invade tissues but instead stays on the surface of the bronchial epithelium
Bordetella pertussis: invasion?
The disease is characterized by paroxysmal coughing (severe coughing fits), followed by a characteristic "whoop" sound as the patient tries to breathe in after a coughing fit.
Pertussis presentation
Tracheal cytotoxin—kills ciliated epithelial cells, impairing clearance of pathogen
Pertussis toxin—ADP-ribosylates the G protein in host cells, which causes increased levels of cAMP, increasing respiratory secretions, making it easier for bacteria to spread (also modulates immunity, making it hard to clear infection)
Behaves similarly to pertussis toxin with respect to cAMP, also acts as hemolysin
Adhesins—target ciliated bronchial epithelia
Bordetella pertussis: 3 virulence factors
Transmitted through respiratory droplets (HIGHLY contagious)
Long incubation period (7-10 days), allowing a long time for asymptomatic spread
Bordetella pertussis: Transmission and incubation period
Catarrhal: 1-2 weeks
common cold symptoms with excessive lacrimation & bloodshot eyes
Paroxysmal: 2-4 weeks
Severe coughing fits with inspiratory “whoop” & impaired mucous clearance, fits may end with exhaustion or vomiting
Convalescent: >1 month
Intensity/frequency of coughing fits diminish, pneumonia may occur. Total duration of symptoms can last 3-4 months.
Bordetella pertussis: three stages of disease
Endemic worldwide with 2-5 year cycles
Humans are the exclusive hosts; half of infected adults may be asymptomatic carriers
1% mortality, mostly in unvaccinated infants
Bordetella pertussis: epidemiology
Diagnosed with PCR (culture is tricky)
Treatment is mainly supportive, abx effective if diagnosed early
Prevention: DTaP vaccine
Bordetella pertussis: dx and treatment
slender & pleomorphic
Gram negative but often stains poorly with traditional Gram techniques
Obligate aerobe
Legionella pneumophila: appearance, Gram, oxygen requirements
primarily exists in natural aquatic environments like freshwater (rivers, lakes) and moist environments, often associated with amoebas
It also forms biofilms in man-made water systems.
Legionella pneumophila: natural habitat
Inhalation of contaminated aerosols
does not spread person to person
Legionella pneumophila: transmission
Inhaled into lungs, taken up by alveolar macrophages
Once inside, actively multiplies within cell (injects effector proteins into host that prevent phagosome-lysosome fusion)
Can also escape host by breaking down cell membrane
Legionella pneumophila: colonization
Legionella pneumophila causes Legionnaires' disease, a severe form of pneumonia characterized by fever, cough, chest pain, shortness of breath, and in some cases, gastrointestinal symptoms like diarrhea.
In severe cases, it can lead to multi-organ failure and death, particularly in individuals who are immunocompromised, elderly, or have chronic lung conditions.
Also causes Pontiac fever, a self-limited flu-like illness with no lung involvement
Legionella pneumophila: causes what diseases?
1-10% in healthy people, higher in immunocompromised
Legionnaires' disease mortality
Freshwater sources like lakes and streams, industrial cooling towers (AC systems), showerheads, hot tubs, misters (like those used in landscaping)
Environmental reservoirs of Legionella
Culture: slow, requires special medium with iron and L-cysteine
Urinary antigen test: Faster than culture but can miss cases caused by L. longbeachae
PCR: can be used if antigen test is inconclusive
Diagnosis of Legionella
Antiobiotics: fluroquinolones (can penetrate macrophages)
Glucocorticoids to reduce inflammation if severe pneumonia is present
Treatment of Legionnaire’s disease