physcial science final
1/297
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
298 Terms
According to the hypothesis of de Broglie, any moving particle has
a wavelength
The original nucleus before decay is known as the:
parent nucleus
Calculate the energy En (in eV) of a hydrogen atom's electron with n = 4.
-0.85 eV
Isotopes of an element differ in their numbers of ____________.
neutrons
When an electron in an atom falls from a higher energy level to a lower one, a ______________ is ______________.
photon; emitted
What is the symbol for the element whose atoms have 3 protons each?
Li
Radioactive decay can occur over multiple decay steps.
True
The half-life for Iodine-131 is 8 days. How many half-lives will have passed after 24 days?
Three half-lives
The nucleus makes up about what percentage of the mass of an atom?
99.9%
How many protons does Radon have?
86
The mass defect for a D-T fusion reaction is calculated as 0.188 u. The MeV energy conversion factor is given as 931 MeV/u. What is the energy released by this fusion reaction?
175 meV
For a large nucleus when the number of protons exceeds 83, the nucleus:
becomes unstable
Who discovered the electron in 1887?
Thomson
Who, in 1925, postulated that matter, as well as light, has properties of both waves and particles?
de Brogile
The average mass of the atoms of an element in naturally occurring samples is called the ______________ mass of the element.
atomic
How many protons are there in an atom of 23/11 Na?
11
After 2 half-lives only _______ of the original amount of the radionuclide remains undecayed.
One quarter
The greater the frequency of light, the greater the ______________ of its photons.
energy
What is the symbol for the element whose atoms have 92 protons each?
U
Calculate the radius in nanometers of the orbit of a hydrogen atom's electron with n = 5.
1.3
Most stable types of nuclei have an ______________ number of protons and an ______________ number of neutrons.
even; even
The atomic number (Z) of an element is equal to the number of ______________ in one of its atoms.
protons
The process in which smaller nuclei combine to form larger nuclei is called nuclear fission
False
What is the symbol of the element that has 10 protons?
Ne
A rock of mass 0.100 kg is thrown with a speed of 50.0 m/s. What is its deBroglie wavelength?
1.33 x 10-34 m
The diameter of an average nucleus is about
10-14 m
Which is not a possible value for the principal quantum numbern?
3/2
When a hydrogen electron is in its ground state, its principal quantum number is
1
The "billiard ball model" of the atom is associated with
Dalton
What is the symbol for the element Actinium?
Ac
Matter waves are also called ___________waves.
deBrogile
Transmutation is the process whereby radioactive nuclei can spontaneously change into nuclei of other elements.
True
Calculate the energy En, in electron volts, of a hydrogen atom's electron with n = 6.
-0.378 eV
Removal of an electron from an atom leaves a
positive ion
The "plum pudding model" of the atom is associated with:
Thomson
The symbol notation now used for elements was developed by
Berzelius
_________ is the source of energy for the Sun and other stars.
Fusion
A quantum is a
discrete amount of energy
For a large nucleus, when the number of protons exceeds 83, the unstable nucleus can undergo:
Radioactive Decay
The process by which a large nucleus splits into smaller nuclei, releasing energy and emitting neutrons, is called:
nuclear fission
How many neutrons are there in an atom of 235/92
143
The lowest energy level of a hydrogen atom (n = 1) is called
the ground state
Light can sometimes be described as a wave, and sometimes as a particle.
True
An exoergic reaction
releases energy
What is the symbol used on the periodic table for the element Rubidium?
Rb
What is the symbol for Silver?
Ag
Calculate the radius in nanometers of the Bohr orbit of a hydrogen atom's electron with n = 6.
1.91 nm
What is the symbol for the element copper?
Cu
How many electrons are there in a neutral atom of 56/26?
26
The development of physics since about 1900 is called
modern physics
The symbol for tungsten is
W
Atoms of which group of elements have seven electrons in the outer shell?
Halogens
Alkaline earth metals lose two valence electrons, meaning they are in:
Group 2A
Which element has the atomic number 9?
F
The horizontal rows of elements in the periodic table are called:
Periods
Which of the following is NOT an ion?
O^2
The Lewis symbol •X• would be characteristic of which group?
2A
How many valence electrons does an atom of phosphorus have?
5
In 1774, ______ discovered the Law of Conservation of Mass.
Laviosier
The vertical columns of elements in the periodic table are called
Groups
The octet rule states that the formula for a compound is derived by
having all atoms or ions get noble gas electron configurations
The symbol for copper is
Cu
What’s the name for a negatively charged ion?
Anion
Polar substances tend to mix well with nonpolar substances.
False
How many valence electrons do the Group 6A elements have?
6
The most abundant element in the universe is
Hydrogen
A compound consists of only magnesium, carbon, and oxygen. If the percentage by mass of Mg is 28.8% and that of C is 14.2%, what’s the percentage by mass of O?
57.0%
If constituent elements are not mixed in the correct proportions then the one element that will be leftover is called the
excess reactant
A compound consists of only magnesium, carbon, and oxygen. If the percentage by mass of Mg is 63.9 and that of C is 12.2%, what’s the percentage by mass of O?
23.9%
A molecule is polar if electrons are attracted to one end of a molecule?
True
Atoms of which group of elements have seven electrons in the outer shell?
Halogens
An atom that has equal amounts of protons and electrons is a ____ ion.
Neutral
The ____ for compounds are written by putting the composing elements’ symbols adjacent to each other, with subscripts indicating the number of atoms of each.
Formulas
Water (H2O) is nonpolar
False
An atom that has more electrons than protons is a ____ ion.
Negative
Having eight electrons in the outer shell is known as the ____ configuration.
Noble gas
The ionic compound made of Fe³+ and CO3²- ions would have the formula
Fe2(CO3)3
Except for hydrogen and helium, atoms in chemical combination tend to have ____ electrons in their outer shell.
8
Suppose 39g of reactant A is mixed with 40g of reactant B. After a chemical reaction takes place between A and B, which statement is correct?
The total mass of products plus any unreacted reactants will be 79g
In a ______, a pair of electrons is shared by two atoms.
Covalent bond
When atoms unite by transferring electrons, they should form
An ionic compound

The following Lewis symbol would be a characteristic of which group?
7A
How many shells in an atom of silicon contains electrons?
3
The _____ for compounds are written by putting the composing elements’ symbols adjacent to each other, with subscripts indicating the number of atoms of each.
Formulas
What’s the formula mass of ammonium phosphide, (NH4)3P?
85.0 u
In a binary compound, the more metallic element is usually named first.
True
The formula mass of water, H2O, is
18 u
The outermost electron shell can hold up to 10 electrons
False
If a neural element has 8 neutrons and 7 electrons, which expression correctly identifies the element?
15/7 N
The two bottom-most rows on the periodic table are called:
Inner transition elements
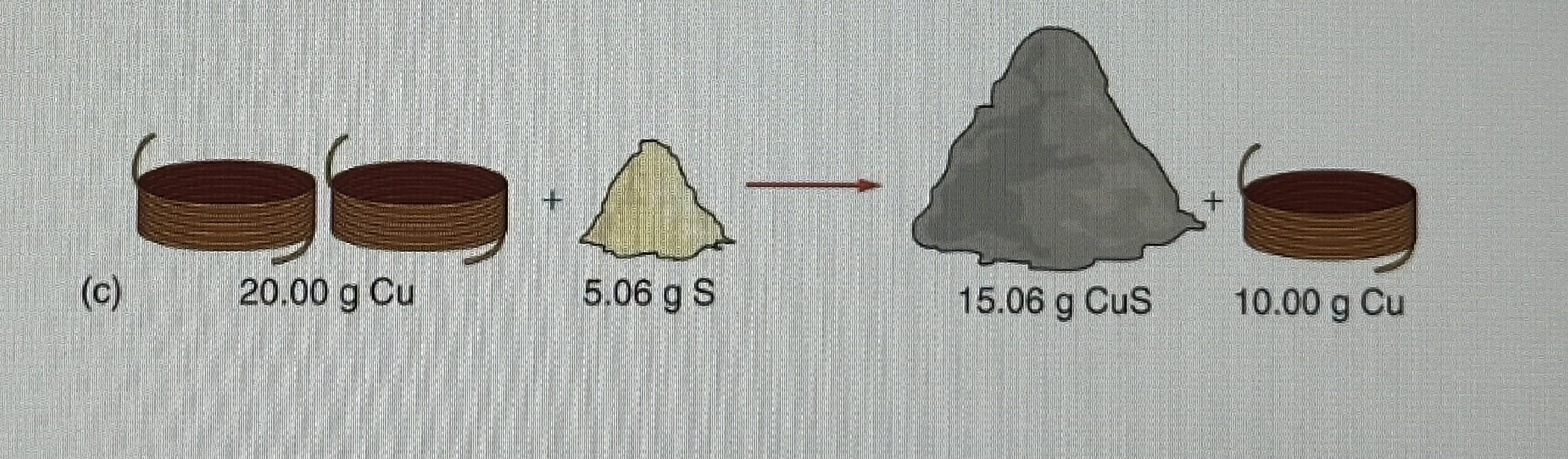
Which reactant below would be described as the express reactant?
Cu
When the atoms of two or more elements react chemically, they usually join together to form
Molecules
The symbol for lead is
Pb
The formula mass of Na2S is
78.1 u
What’s the formula of a compound formed with the ions M²+ and X²-
MX
How many elements occur naturally on Earth?
88
If constituent elements are NOT mixed in the correct proportions, then the one element that’s used up completely is called the:
Limiting reactant
Which of the following Period 4 elements has the largest atom?
Ca
The element potassium should produce compounds most similar to use those of
Na
Avogadro's number has a numerical value of
6.02 × 10²³