Acids and Bases Chemistry Test
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
what is a A Brønsted–Lowry acid and a A Brønsted–Lowry base
A Brønsted–Lowry acid donates a proton H+.A Brønsted–Lowry base accepts a proton H+
Difference between base and an alkali
Alkalis release OH⁻ ions directly in water and are soluble in water
ie. NaOH → base and alkali
CuO → base but not an alkali
how to deduce the Brønsted–Lowry acid and base in a reaction
look for proton
who lots the proton (acid)
who gained the proton (base)
how to find conjugate acid and base
think does an acid donate or accept the proton.
Conjugate acid of a base (adds a H+) ie. NH3 to NH4+
Conjugate base of an acid (remove one H⁺) H2SO4-
make sure to adjust the charge!
species that can act as both Brønsted–Lowry acids and bases + some can lose multiple H ions why is this important?
H₂O
HCO₃⁻
HSO₄⁻
H₂PO₄⁻
Monoprotic, Diprotic, Triprotic (how many H+ it can lose) makes it even more acidic
how to formulate an equation to show acod-base reaction for amphiprotic species
Identify the amphiprotic species (can donate or accept H⁺).
Decide its role:
If reacting with a base → it acts as an acid (donates H⁺).
If reacting with an acid → it acts as a base (accepts H⁺).
Show the H⁺ transfer clearly in the equation.
Write the products: the conjugate acid or conjugate base.
Check charges and formulas are correct.
relationship between ph and H+ concentration (equation)
pH = –log10[H+]/ [H+] = 10–pH Where [H+] is the hydrogen ion concentration in mol dm3
Why as a log scale?
Each unit 10x change Water based solutions vary over a huge range. Logarithm compresses the range.
How to perform calculations involving the logarithmic relationship between pH and [H+].
Ensure H+ is in mol dm-3
Adjust for any dilutions
sub into equation
How to measure PH
1) Indicators (color change)
Litmus: red in acidic solutions, blue in basic solutions (very rough).
Universal indicator: gives an approximate pH by matching the solution color to a color chart. (0 is red purple is 14)
2) pH probe (pH meter)
Gives a numerical pH value and is typically more precise than indicators.
Must be calibrated using buffer solutions (commonly pH 4.00, 7.00, 10.00).
Kw is ? and where does it come from?
Kw=[H⁺][OH⁻]
ion product constant of water.
It comes from water partially dissociating:
H₂O ⇌ H⁺ + OH⁻\text{H₂O ⇌ H⁺ + OH⁻}H₂O ⇌ H⁺ + OH⁻
at 25°C, Kw=?
1.0×10−14
Strong acid vs Weak acid – what’s the difference?
Strong acid: completely ionizes in water → all H⁺ released
Examples: HCl, HNO₃, H₂SO₄ typically hydrogen halides or hydrogen polyatomic
forward arrow
Weak acid: partially ionizes → only some H⁺ released
Examples: CH₃COOH, H₂CO₃
reversible arrow
Strong base vs Weak base – how do they behave?
Strong base: fully dissociates in water → all OH⁻ released
Examples: LiOH, NaOH, KOH, Ba(OH)2 normally group 1 hydroxides
forward arrow
Weak base: partially reacts with water → some OH⁻ formed
Examples: NH₃, CH₃NH₂
reversible arrow
where do strong/weak acids/bases lie on ionization equilibrium
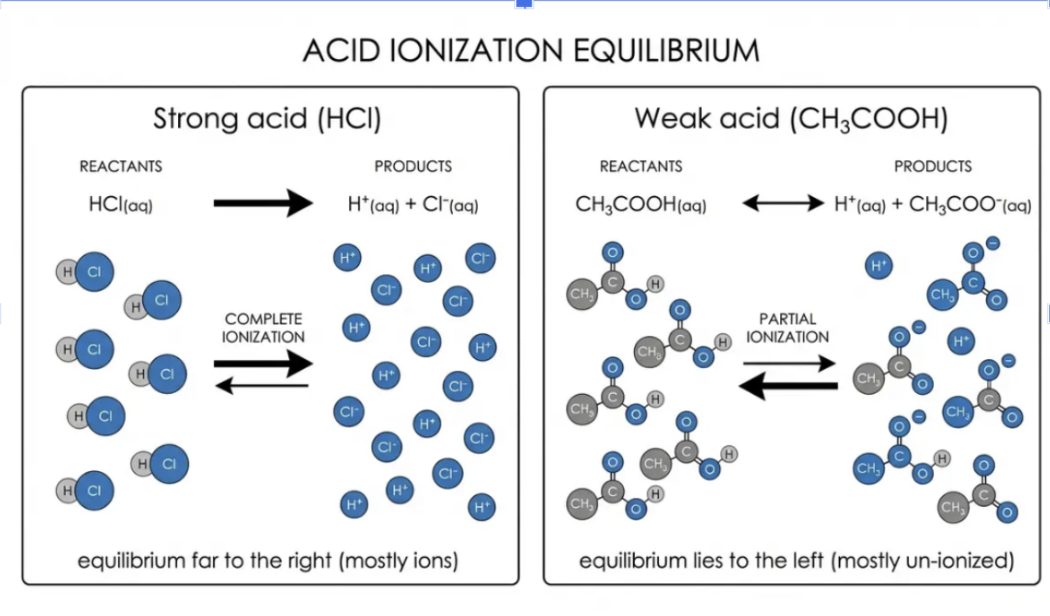
difference between strength and concentration
Strength: how much a substance ionizes (an intrinsic property of the substance).
Concentration: how much solute is dissolved per volume (a property of how you prepared the solution).
neutralization reactions with metal oxides, metal hydroxide, metal carbonate, metal hydrocarbonate
Acid + Metal Oxide → Salt + H₂O
Acid + Metal Hydroxide → Salt + H₂O
Acid + Metal Carbonate → Salt + H₂O + CO₂
Acid + Metal Hydrogencarbonate → Salt + H₂O + CO₂
Strong Acid and Strong Base pH curve
Starts very low pH (strong acid)
Sharp rise at equivalence point
Equivalence point ≈ pH 7
Ends very high pH (strong base)
Curve is S-shaped / sigmoidal
x-axis: volume of titrant added (usually in cm3or mL)
y-axis: pH of the mixture in the flask
Strong and weak acids and bases can be distinguished by:
pH measurement/indicator
ii conductivity
iiirate of reaction with metals, metal oxides, metal hydroxides, metal hydrogencarbonates and metal carbonates.