Properties of Water
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
Adhesion
The property of water that allows it to "stick" to other substances
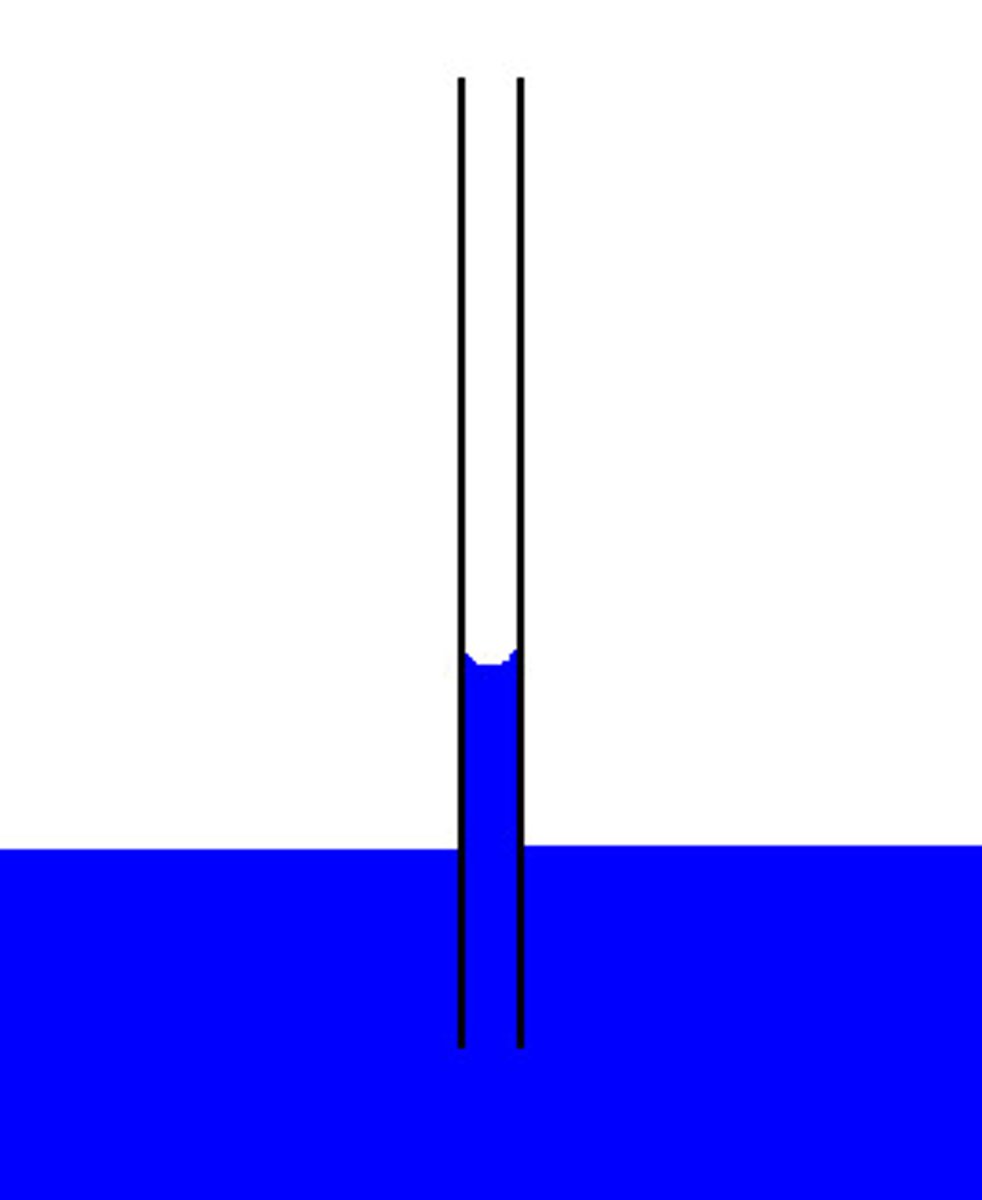
Cohesion
the property of water responsible for water's ability to stick to other water molecules

Universal Solvent
used to describe water because it dissolves many substances

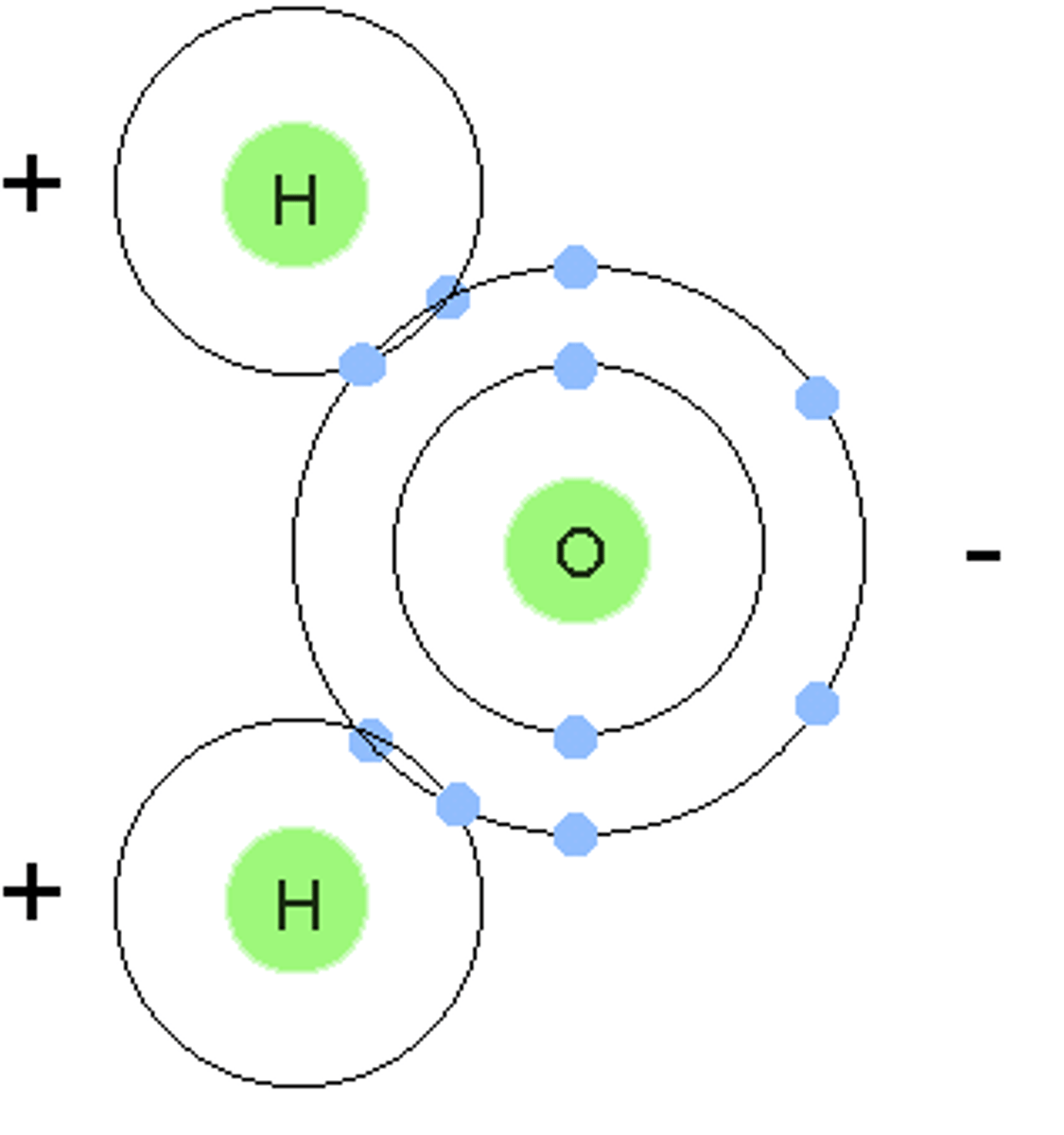
Polar molecule
Has a slight positive and slight negative charge
High heat capacity
What property of water explains why coastal areas climates do not fluctuate as severely as further inland?
Solute
This gets dissolved into a solvent
Solvent
This dissolves substances
Ice Floats because..
It is less dense as a solid
Solution
Homogeneous Mixture that is a liquid
Homogeneous Mixture
Mixture where things combined uniform throughout
Heterogeneous Mixture
Mixture where things are combined unevenly
Suspension
Type of liquid where components can be mixed evenly but will settle after time
Hydrogen bond
A weak bond due to positive nature of Hydrogen
Ionic Bond
Giving and taking of electrons
Surface Tension
The top of water is harder to break through because of hydrogen bonds
Capillary Action
When cohesion and adhesion are working together to pull water up a tube-like structure