AS CHEMISTRY PAST PAPERS
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
Example 10
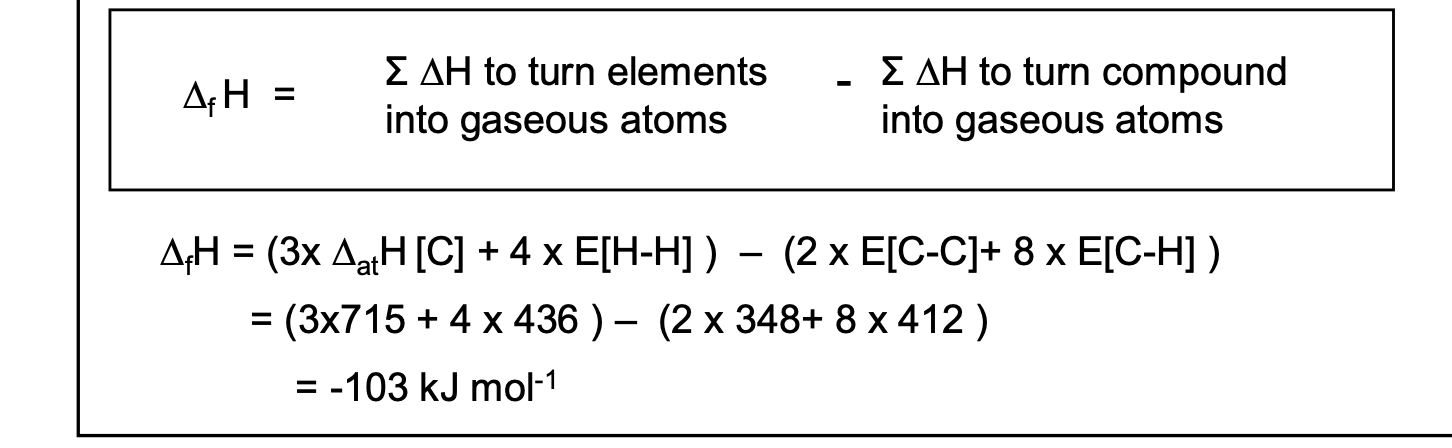
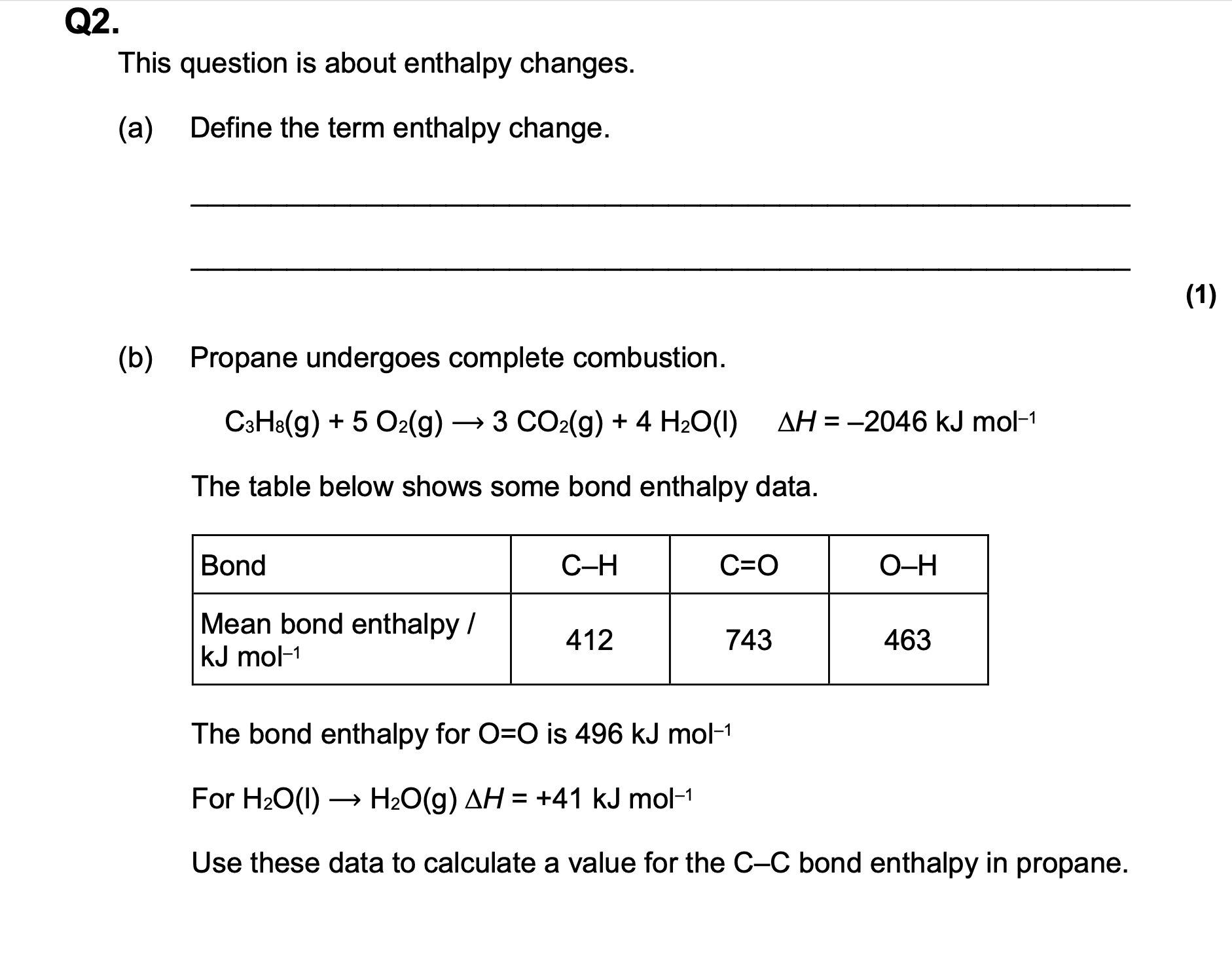
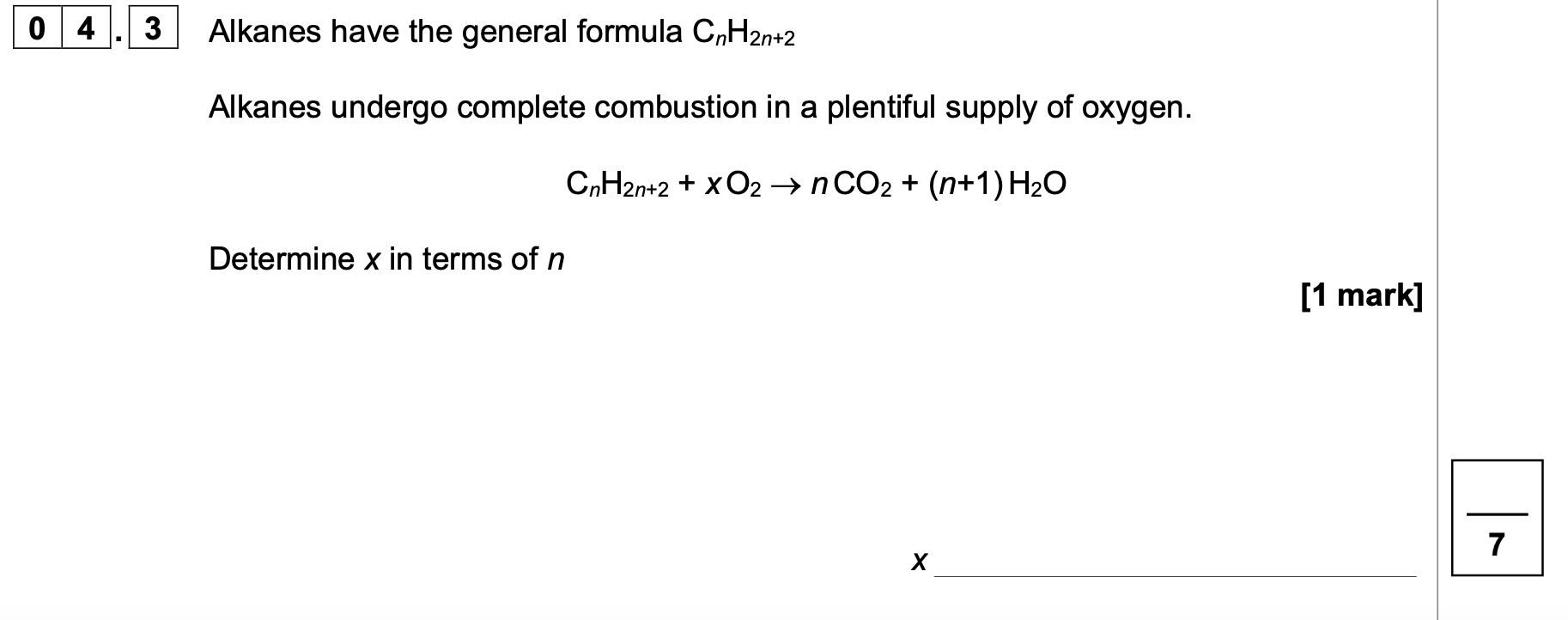
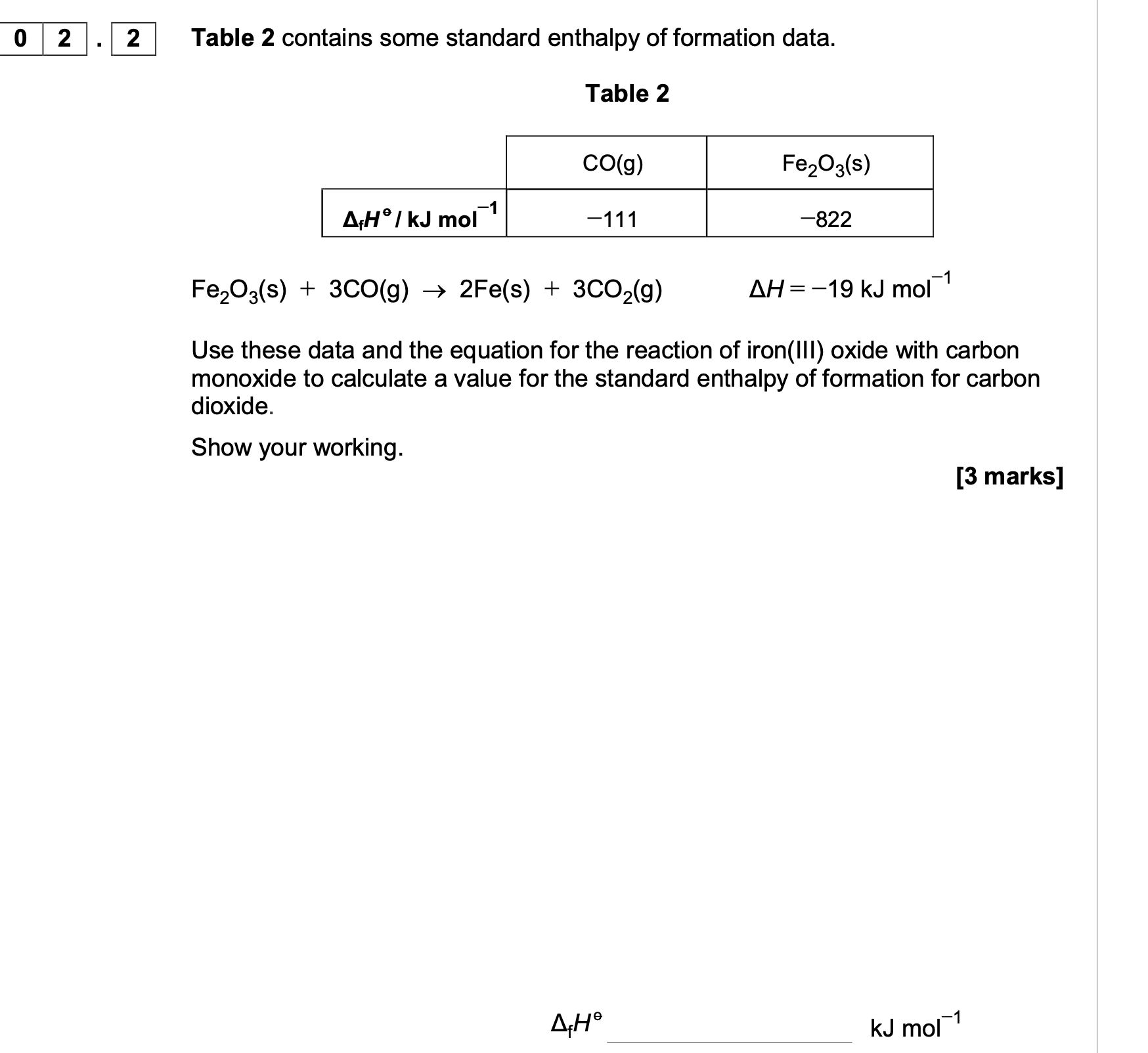
Calculate ΔfH for propane, C3H8(g), given the following data.
C(s) —> C(g) ΔatH = 715 kJ mol-1
3C (s) + 4H2 (g) —→ C3H8(g),
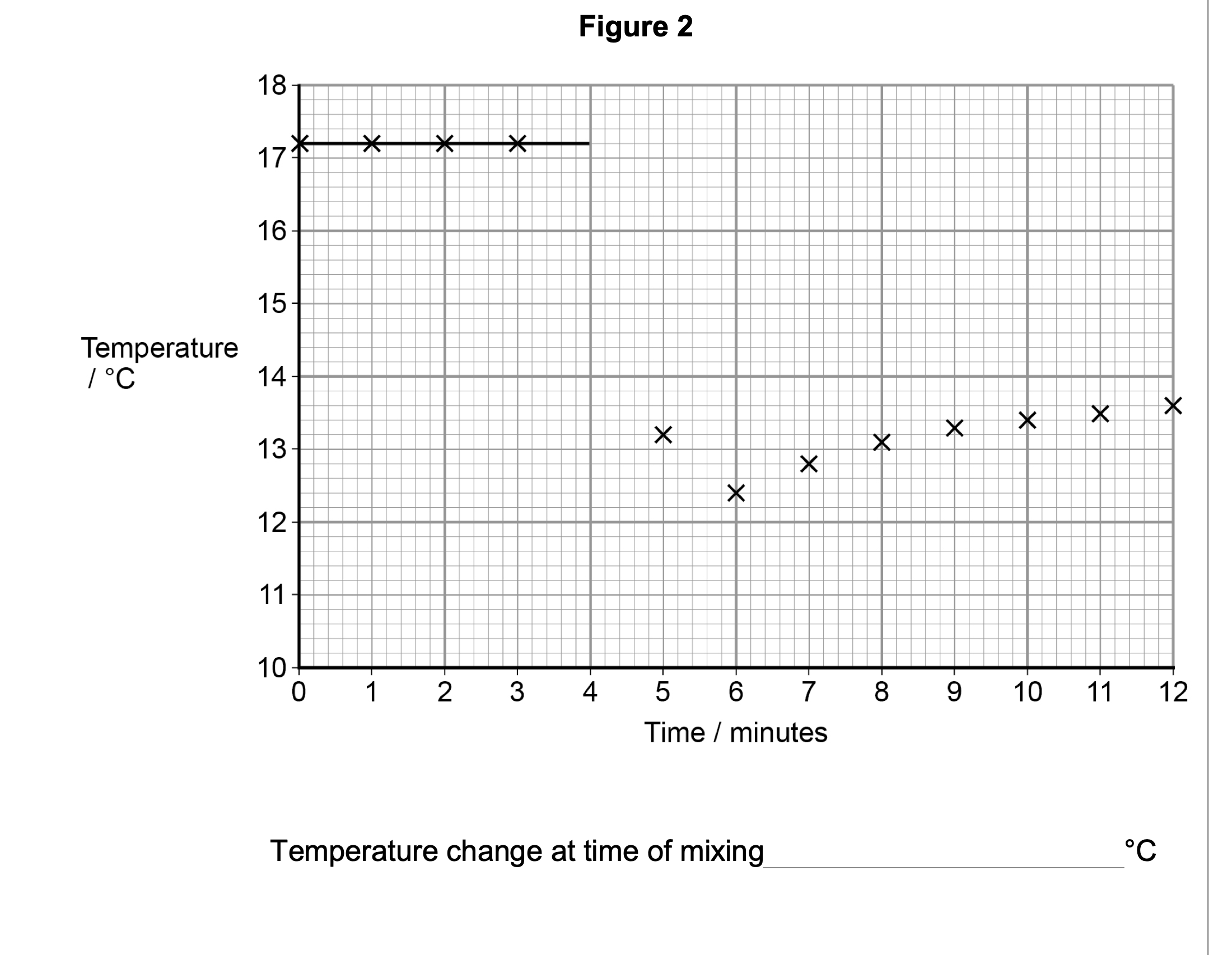
A student recorded the temperature of aqueous ethanoic acid in a polystyrene cup for
three minutes.
At the fourth minute, the student added sodium hydrogencarbonate.
The student stirred the mixture and carried on recording the temperature every minute
for several minutes.
The student’s measurements are shown in Figure 2.
A best-fit line showing the temperature before mixing has been drawn.
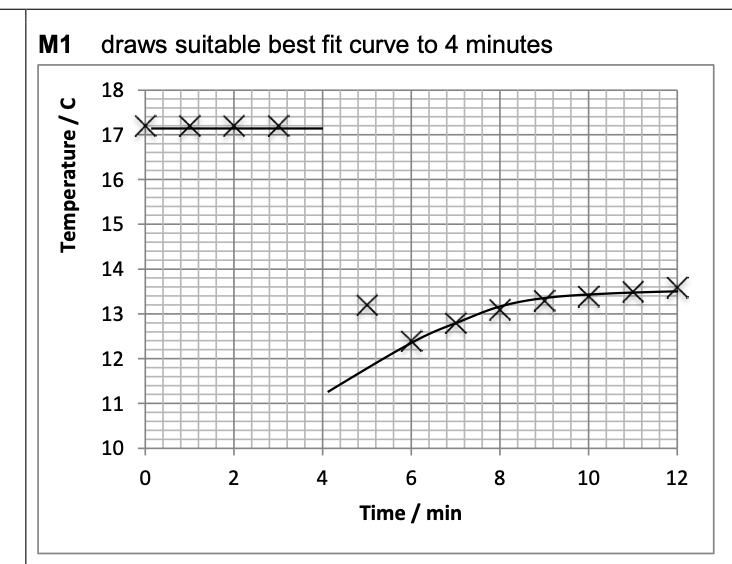
Draw an appropriate best-fit line on Figure 2 and use it to find the temperature
change at the time of mixing.
(17.2 – value read from graph line at 4 minutes) 0.2
( C)
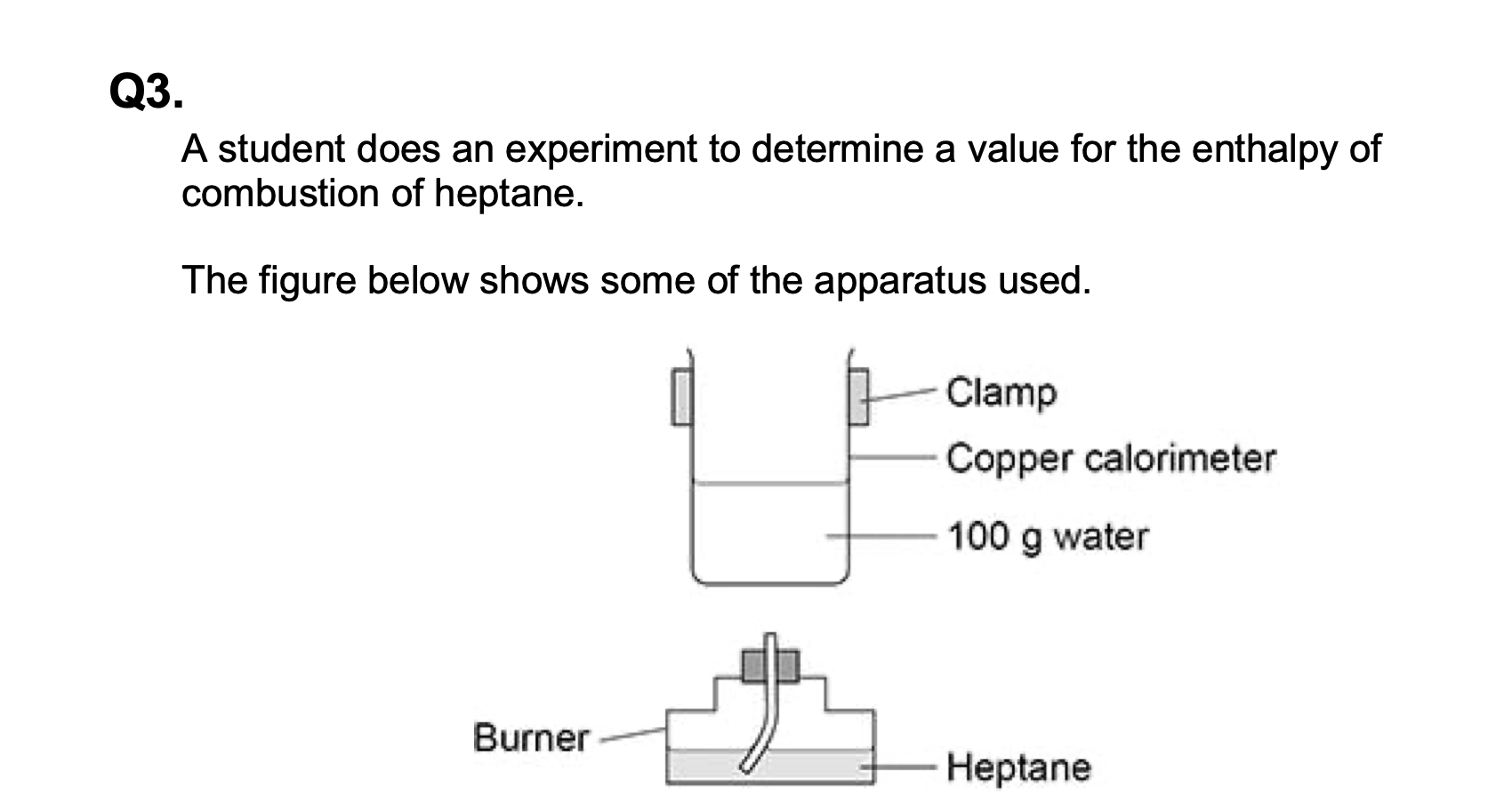
(b) The student considered using a glass beaker on a tripod and gauze instead
of the clamped copper calorimeter.
Suggest two disadvantages of using a glass beaker on a tripod and gauze
Glass is a poorer conductor than copper
M1
M2
M1
Tripod and gauze would reduce heat transfer
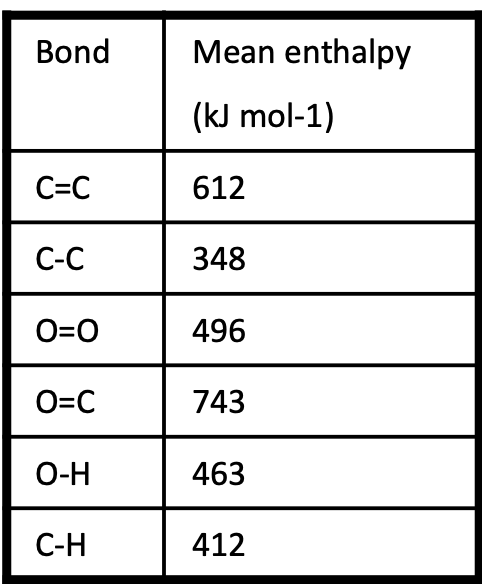
Explain why the value given for the O=O bond enthalpy in part (b) is not a
mean value.
Oxygen / O2 is the only substance that has O=O bond
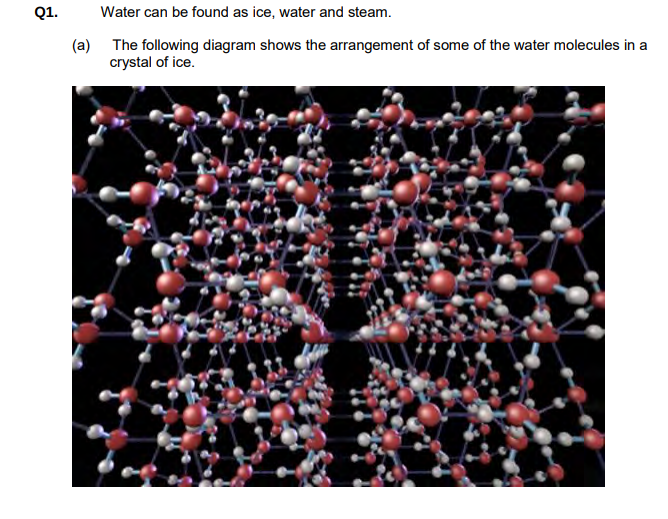
With reference to the structure shown above give one reason why ice is less dense than water.
Water or H2O or molecules (in ice) are held further apart (than in liquid water)/(more) space/gaps/holes in structure/Water or H2O or molecules (in ice) are more spread out
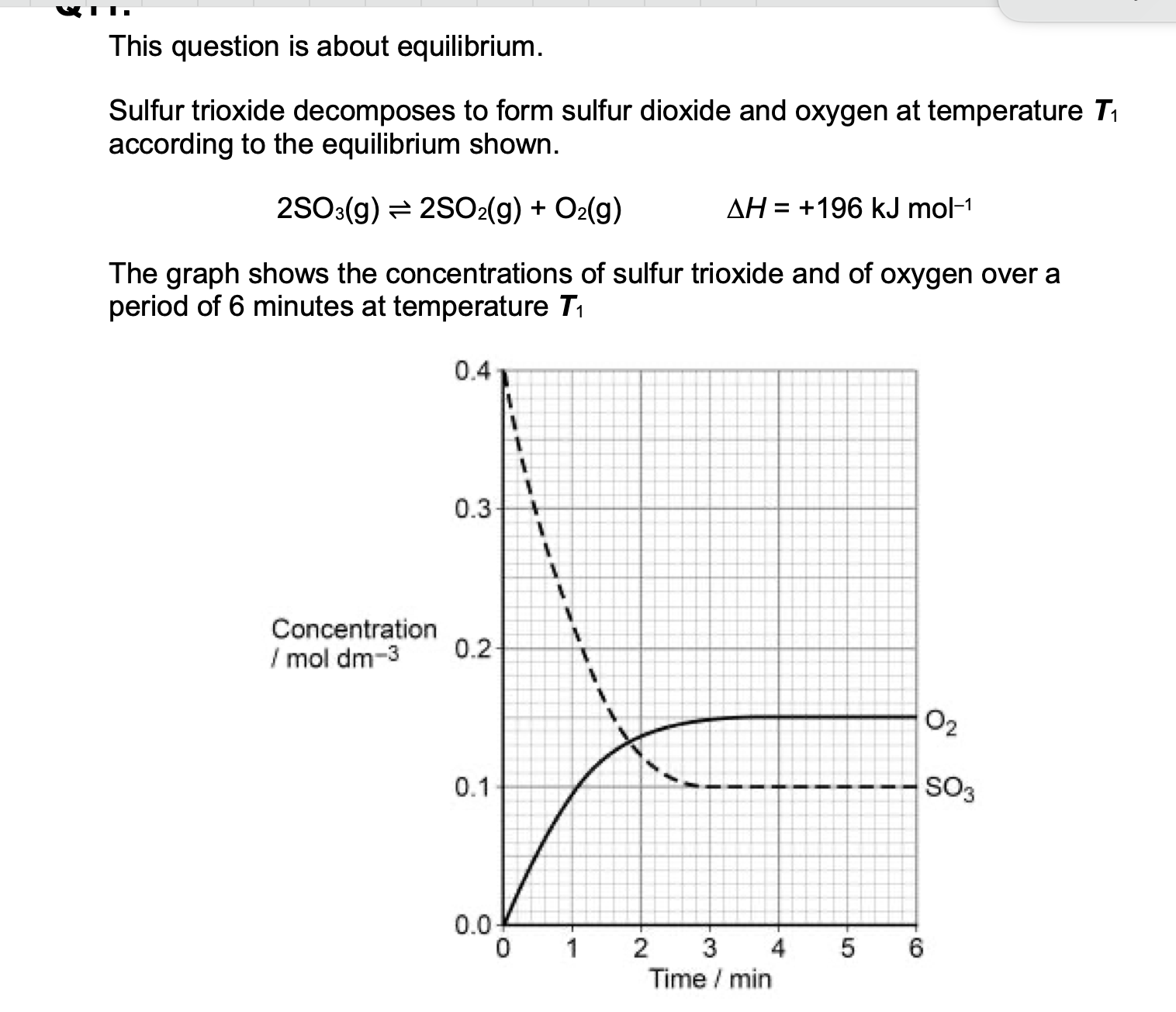
(c) The temperature of the mixture was changed to T2 and the mixture left to
establish a new equilibrium.
In the new equilibrium mixture the concentration of sulfur trioxide was
found to be 0.07 mol dm–3
Deduce which of T1 and T2 is the higher temperature.
Explain your deduction.
Higher temperature _____________
Explanation
_________________________________________________________
___________________________________________________________
___________________________________________________________
___________________________________________________________
______________________________________________
T2 (Not worth a mark alone)
T1, CE=0
Equilibrium has moved / shifted to RHS/forward in endothermic direction
Both RHS / forward and endothermic needed
Equilibrium has opposed the increase in T / Equilibrium moves to decrease
the T
Not just to oppose the change
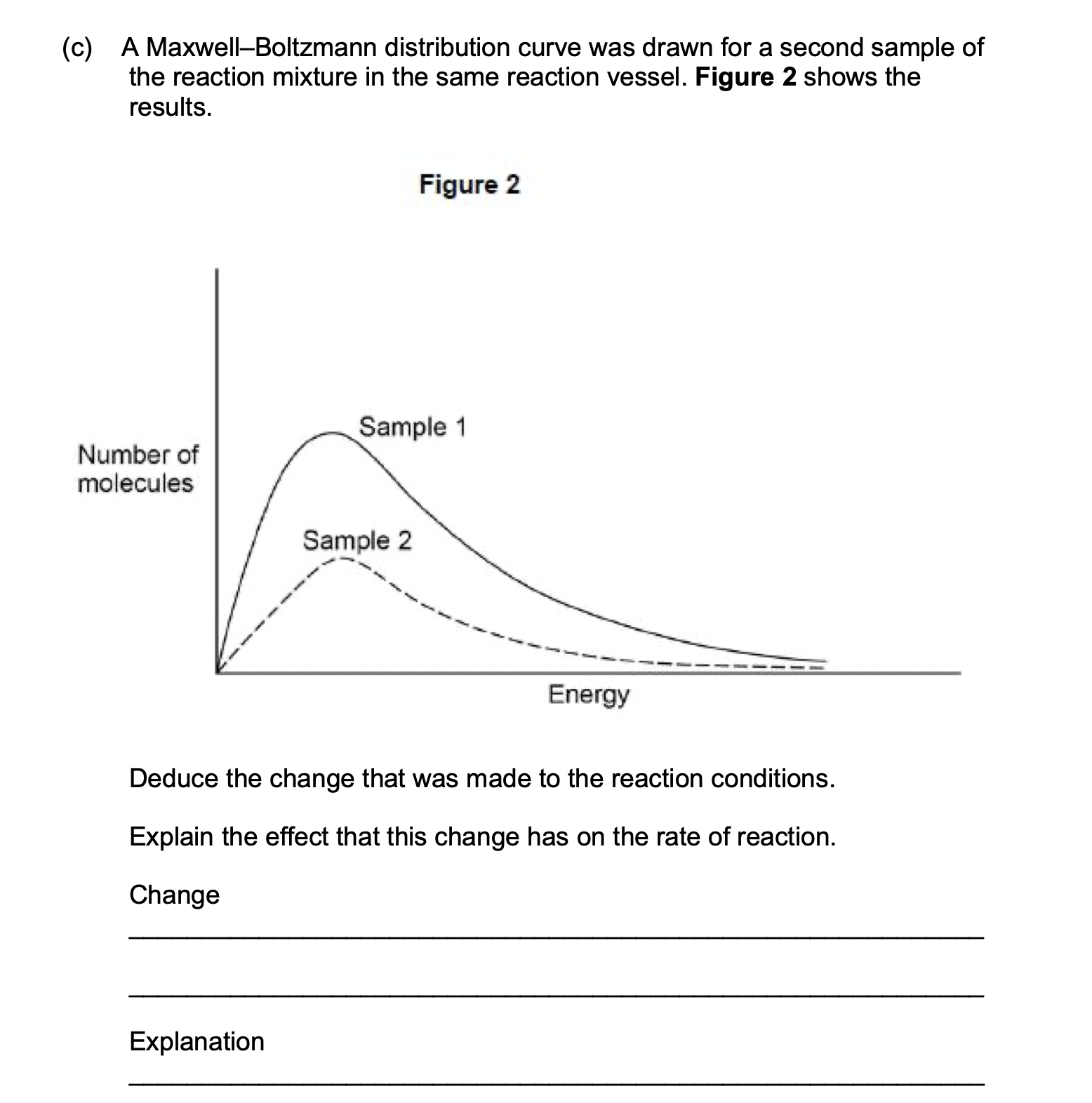
Q10.
Nitryl chloride reacts with nitrogen monoxide according to the equation:
ClNO2(g) + NO (g) ⟶ NO2(g) + ClNO(g)
The Maxwell–Boltzmann distribution curve in Figure 1 shows the distribution of
molecular energies in 1 mol of this gaseous reaction mixture (sample 1) at 320
K.
The amount of gas present (or number of molecules) has been reduced /
or the pressure has been reduced
1
Rate of reaction decreases (no mark)
Particles are spread further apart
1
Fewer collisions between gas particles so fewer successful collisions
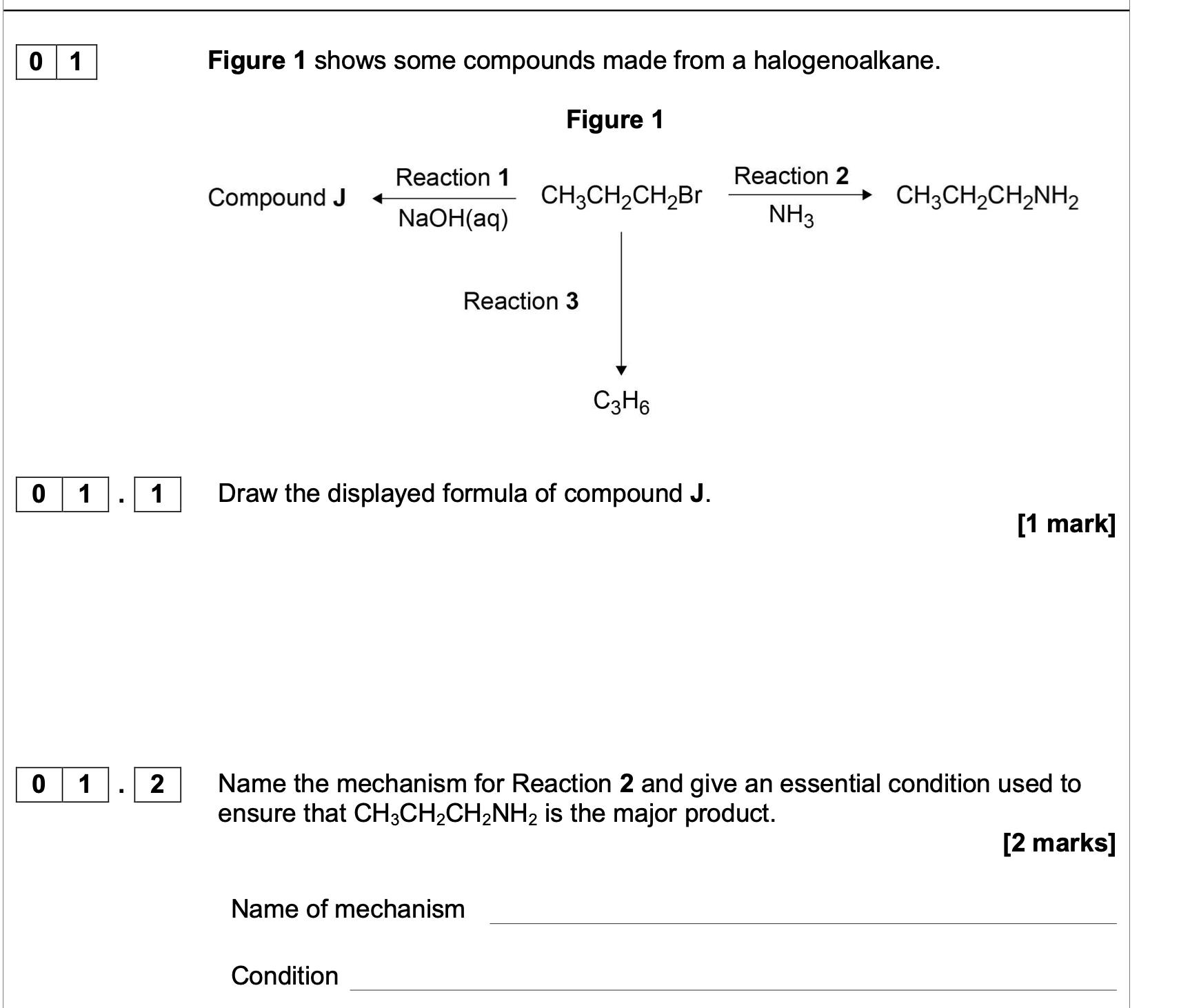
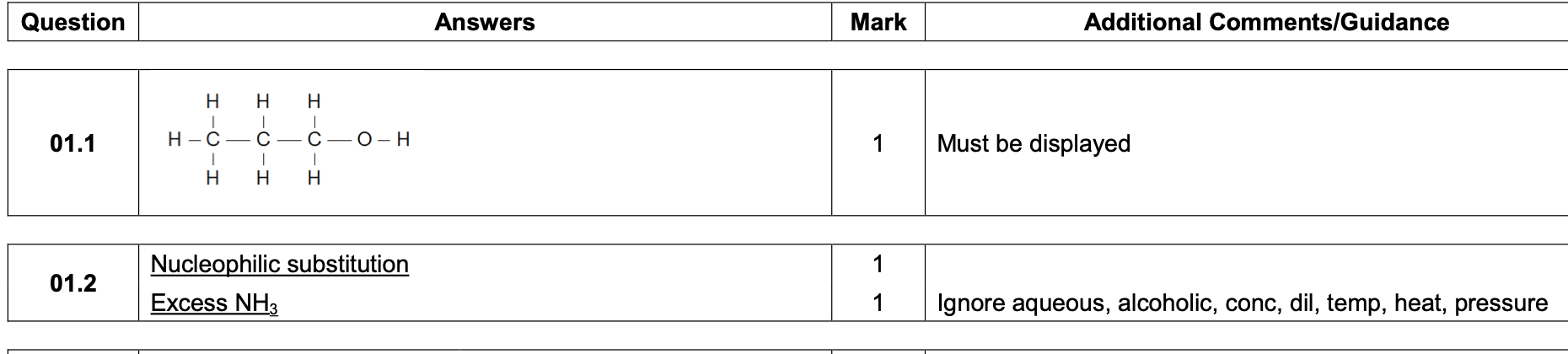
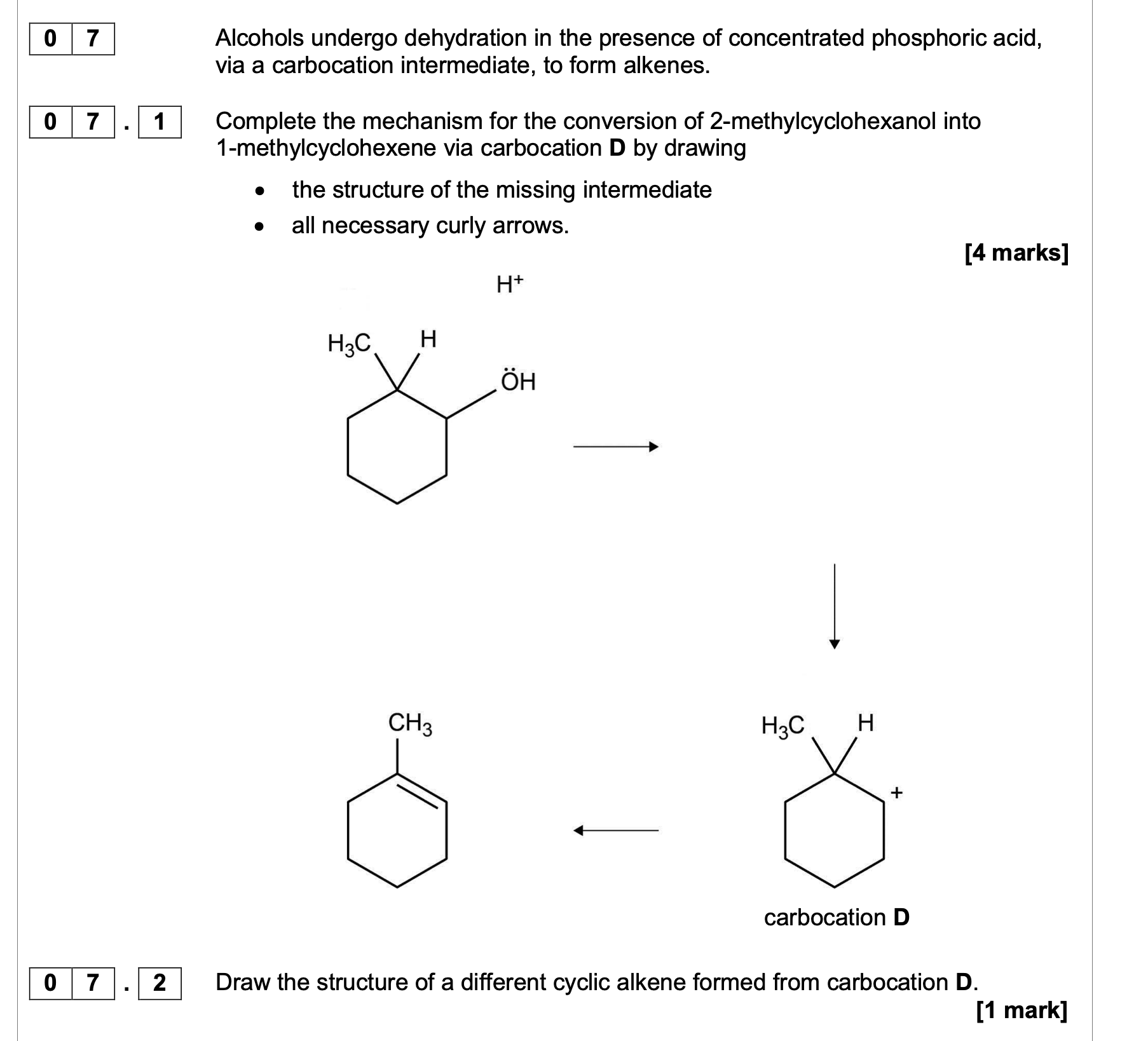
![<p><strong>0 1 . 4 </strong>When Reaction<strong> 2</strong> is carried out under different conditions, a compound with</p><p class="p1">molecular formula C<span>9</span>H<span>21</span>N is produced.</p><p class="p1">Draw the skeletal formula of the compound.</p><p class="p1">Identify the functional group in the compound including its classification.</p><p class="p1"><strong>[2 marks]</strong></p><p class="p1">Skeletal formula</p><p class="p1">Functional group including classification</p>](https://knowt-user-attachments.s3.amazonaws.com/27e1c713-aec7-43e7-8016-df90bd0d9e03.png)
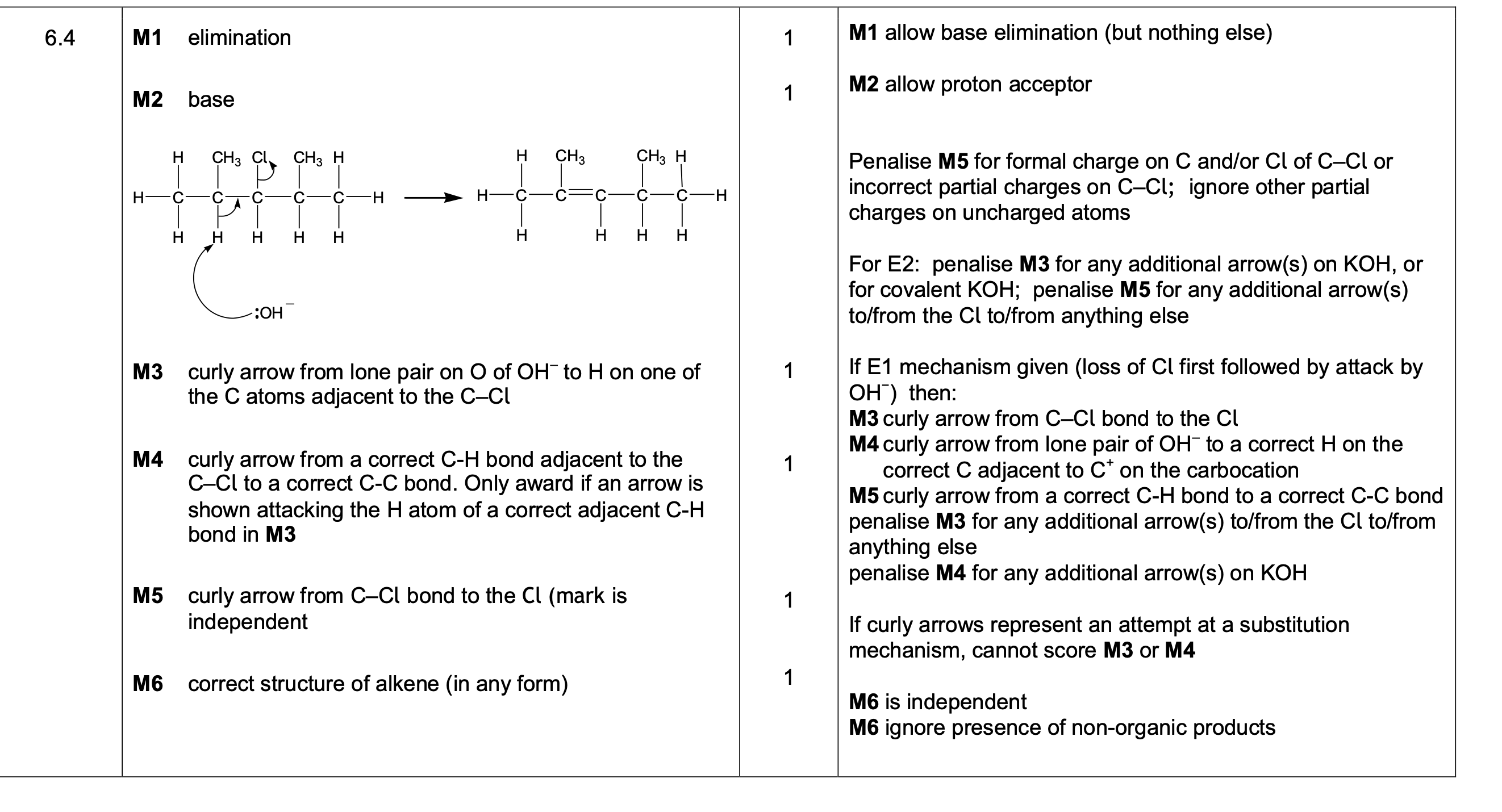
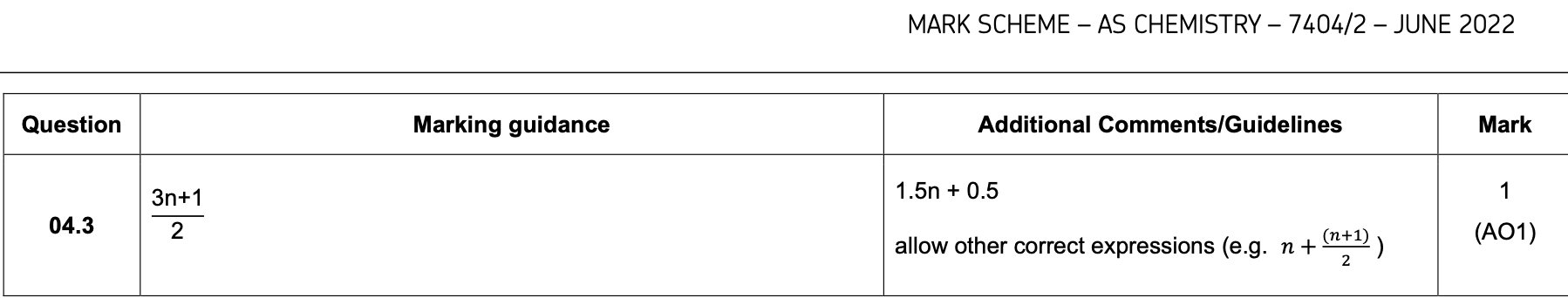
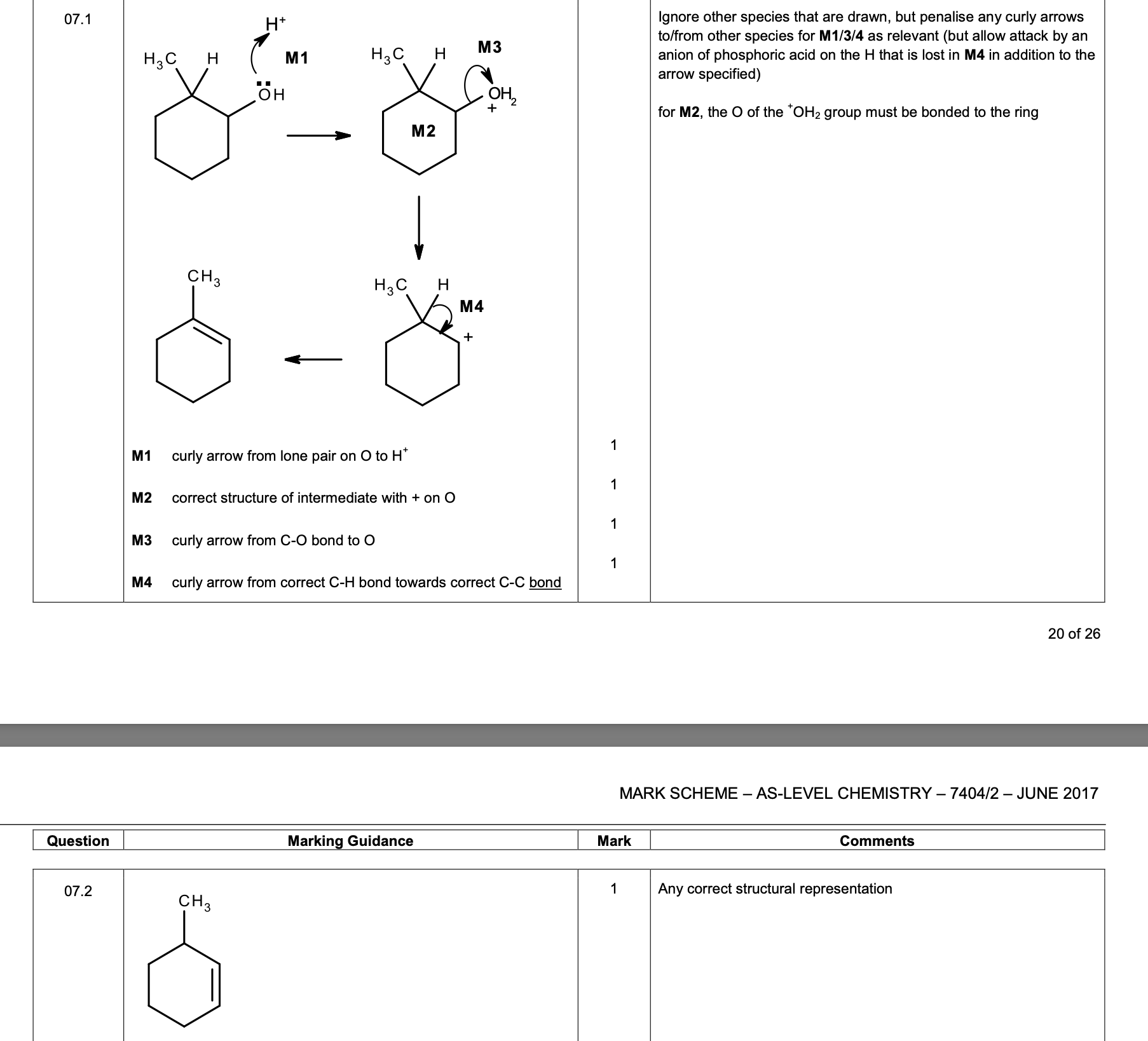
0 1 . 4 When Reaction 2 is carried out under different conditions, a compound with
molecular formula C9H21N is produced.
Draw the skeletal formula of the compound.
Identify the functional group in the compound including its classification.
[2 marks]
Skeletal formula
Functional group including classification


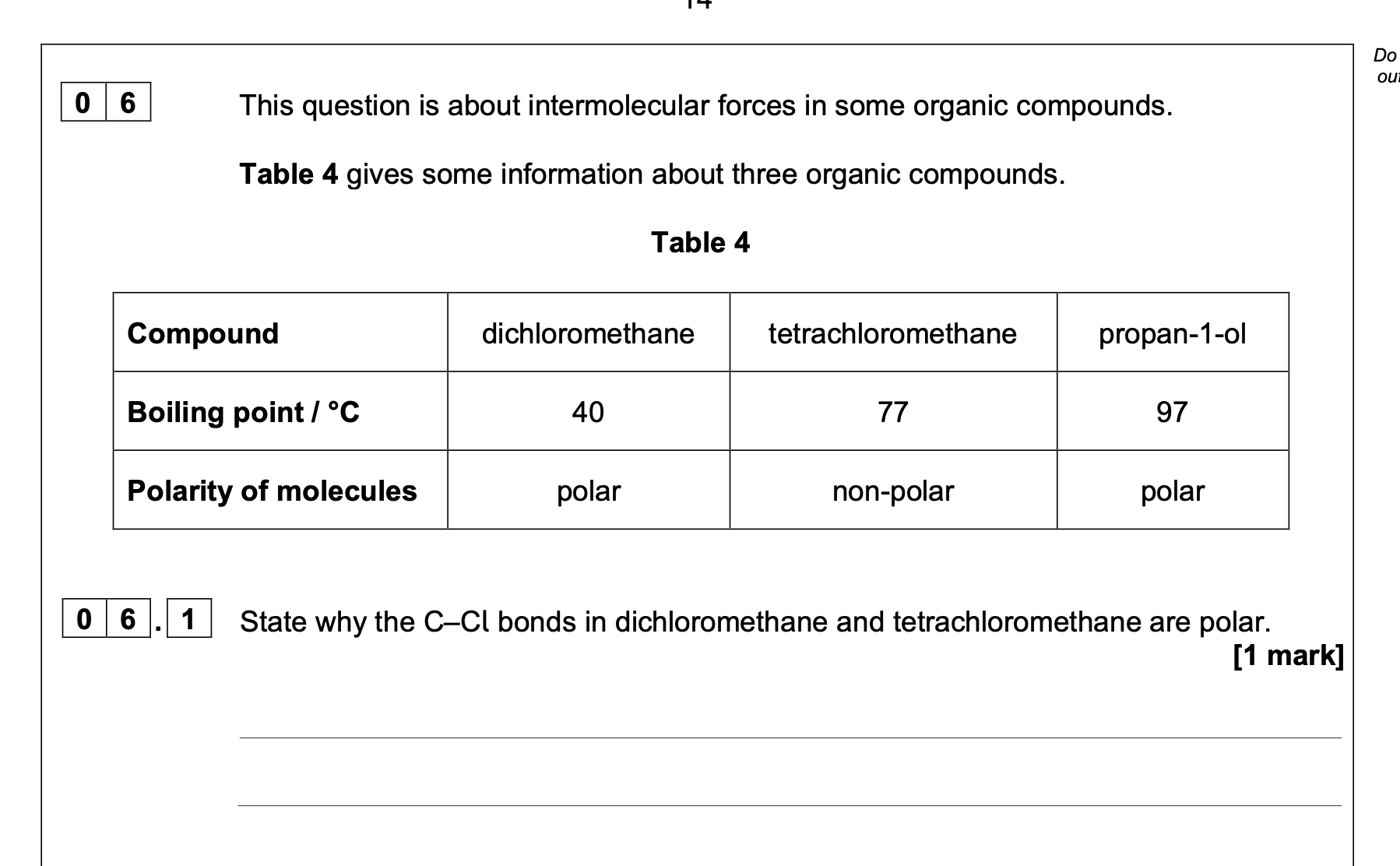
Cl is more electronegative (than C) or
C and Cl have different electronegativities
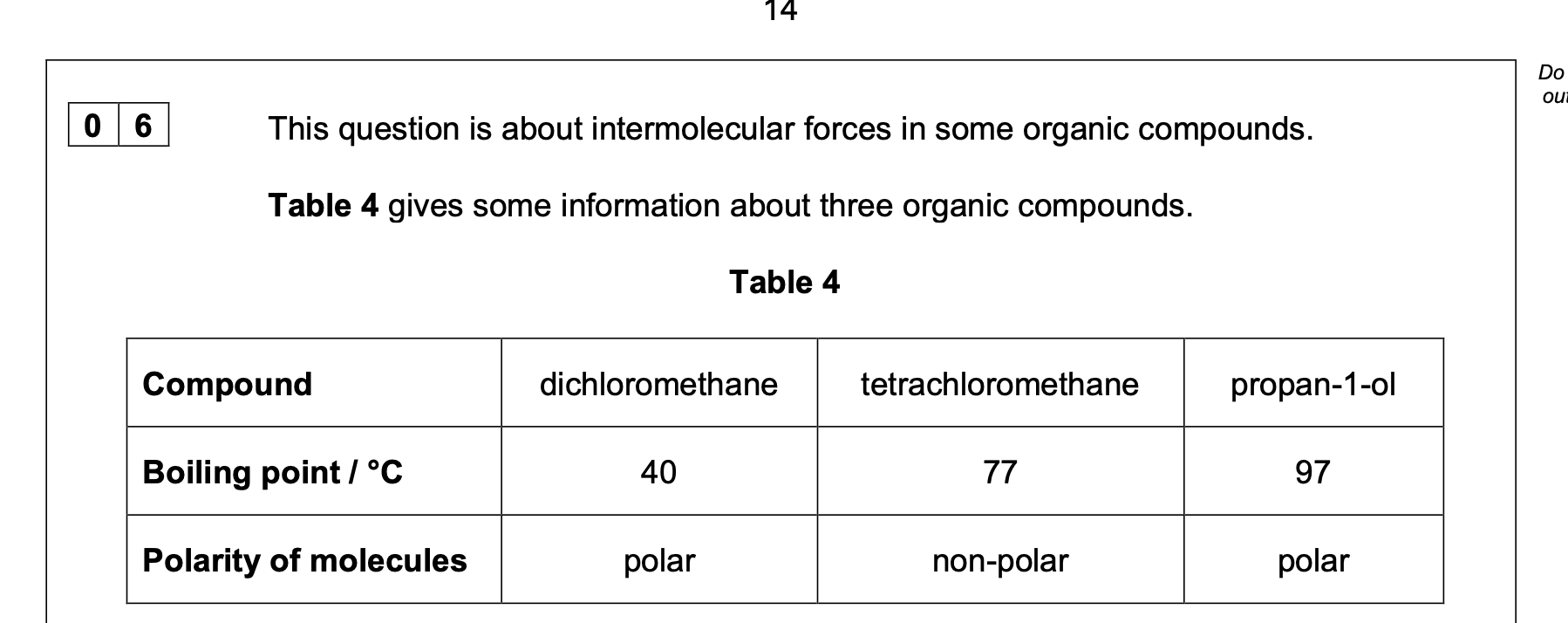
0 6 . 2 Suggest why tetrachloromethane molecules are non-polar.
idea that dipole moments (or dipoles) cancel out (due to symmetry
Allow polar bonds / polarities cancelling out
0 6 . 3 Explain why tetrachloromethane has a higher boiling point than dichloromethane.
M1 M2 van der Waals' forces between molecules in CCl4 stronger than
(combined van der Waals' and) dipole-dipole forces between
molecules in CH2Cl2
as CCl4 has (many) more electrons than CH2Cl2
M1 must refer to the forces being between
molecules at some point
NOT M1 for any reference to bond breaking
NOT M1 for any reference to incorrect
intermolecular forces
Allow London forces or temporary (induced) dipole-
dipole forces for van der Waals' forces
For M2, allow CCl4 has higher mass or higher Mr or
bigger than CH2Cl2
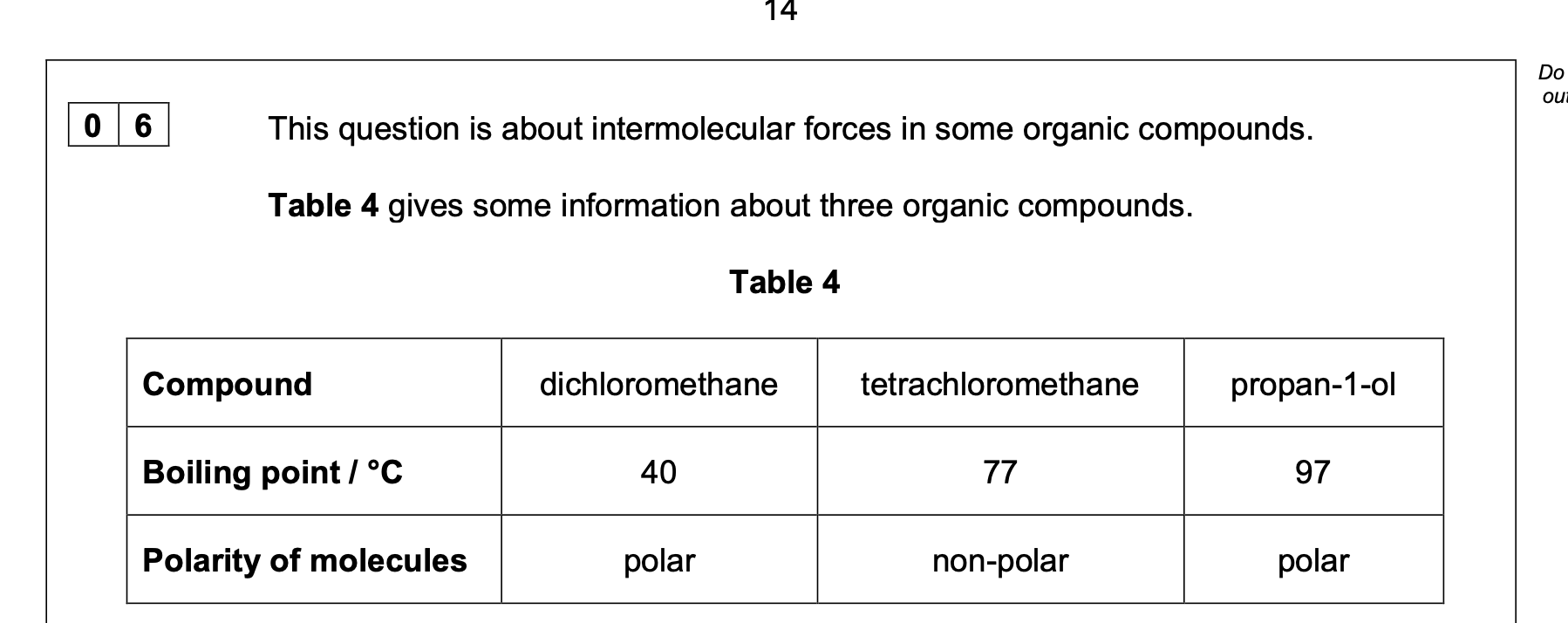
The distillate is collected in the range 77–82 °C
Explain why the water should enter the condenser at the bottom and not at the top
Marking guidance idea of ensuring condenser fills with water
idea that condenser is cool(er) or
ensuring (more) vapour condenses

Oxidation half equation
2 I– → I2 + 2 e–
Reduction half equation
2 IO3– + 12 H+ + 10 e– → I2 + 6 H2O
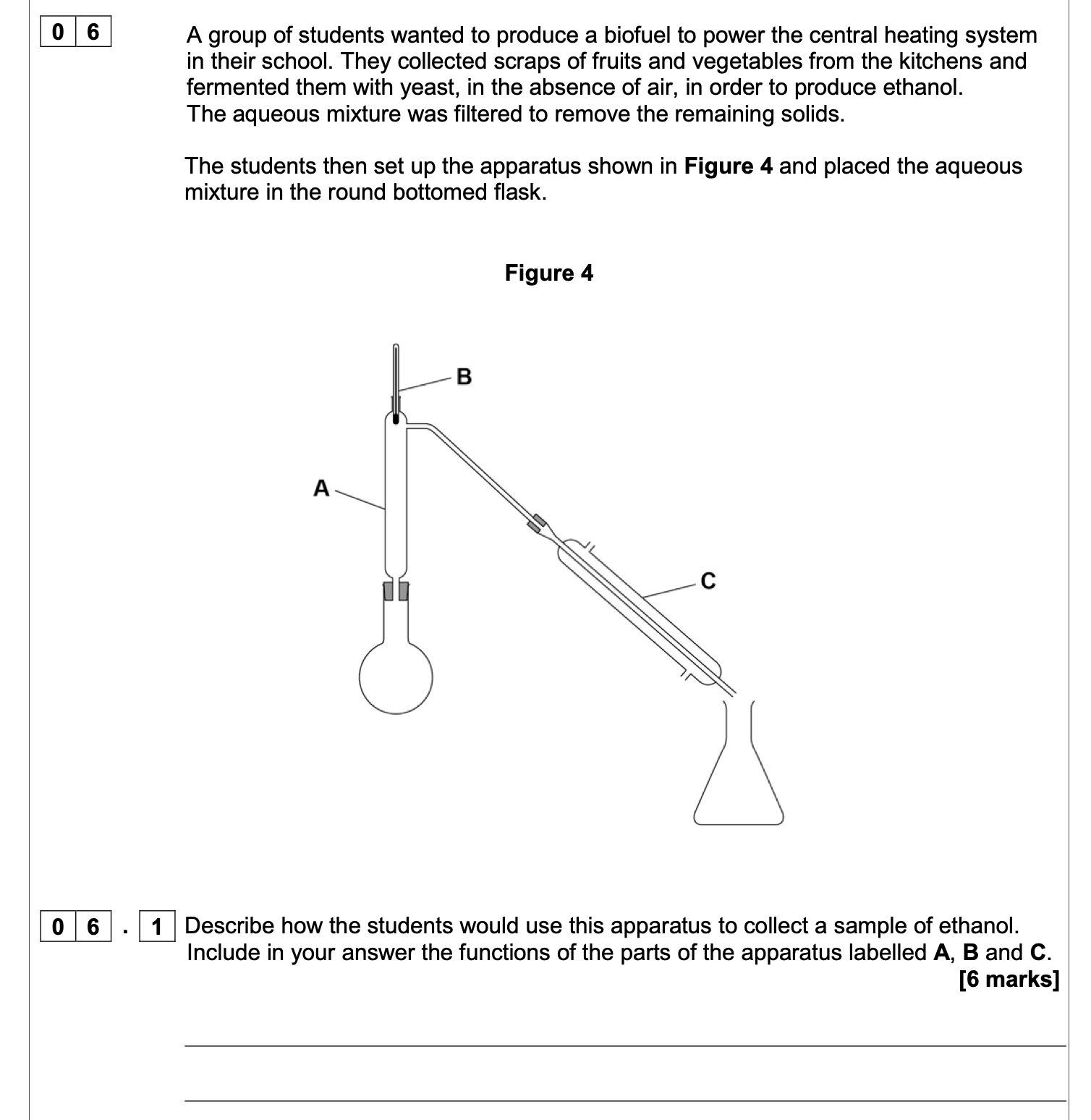
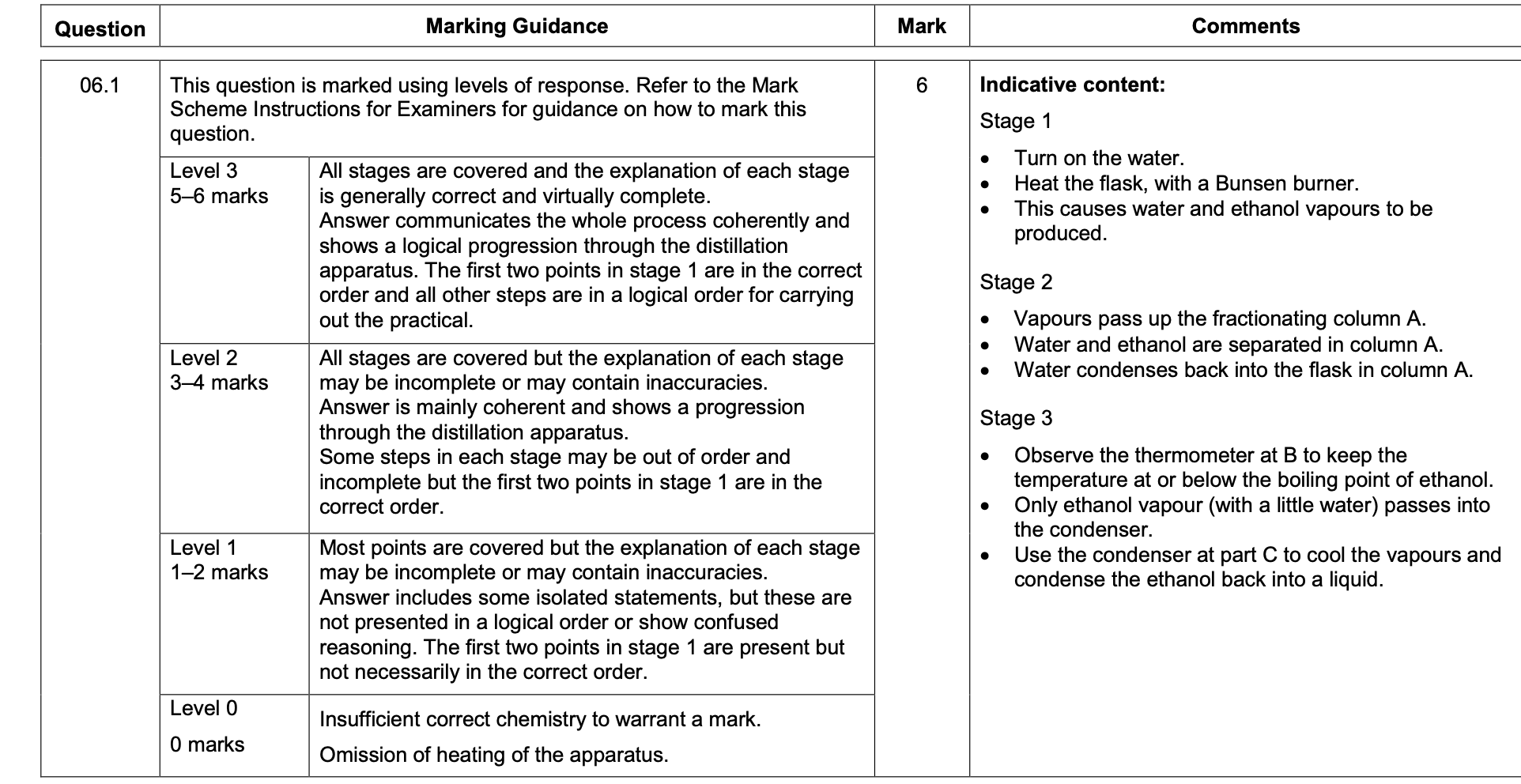
Give two reasons why the apparatus shown in above figure is not safe.
M1 flask not clamped
allow only the condenser is clamped
1
M2 sealed system / bung in condenser
allow explanation of effect of bung being there e.g.
pressure build up
not reference to incorrect water direction
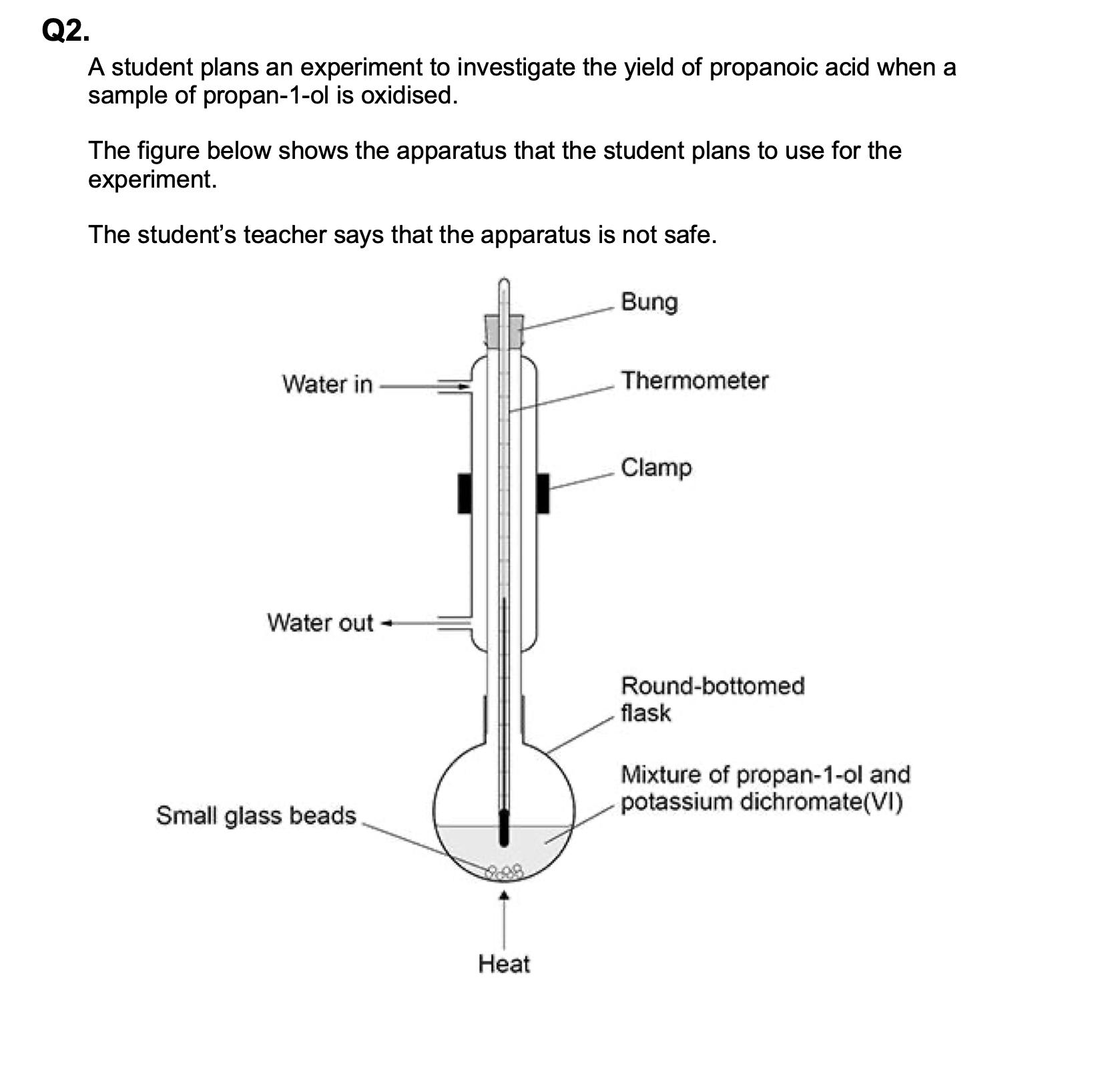
Give one additional reagent that is needed to form any propanoic acid.
dilute sulfuric acid
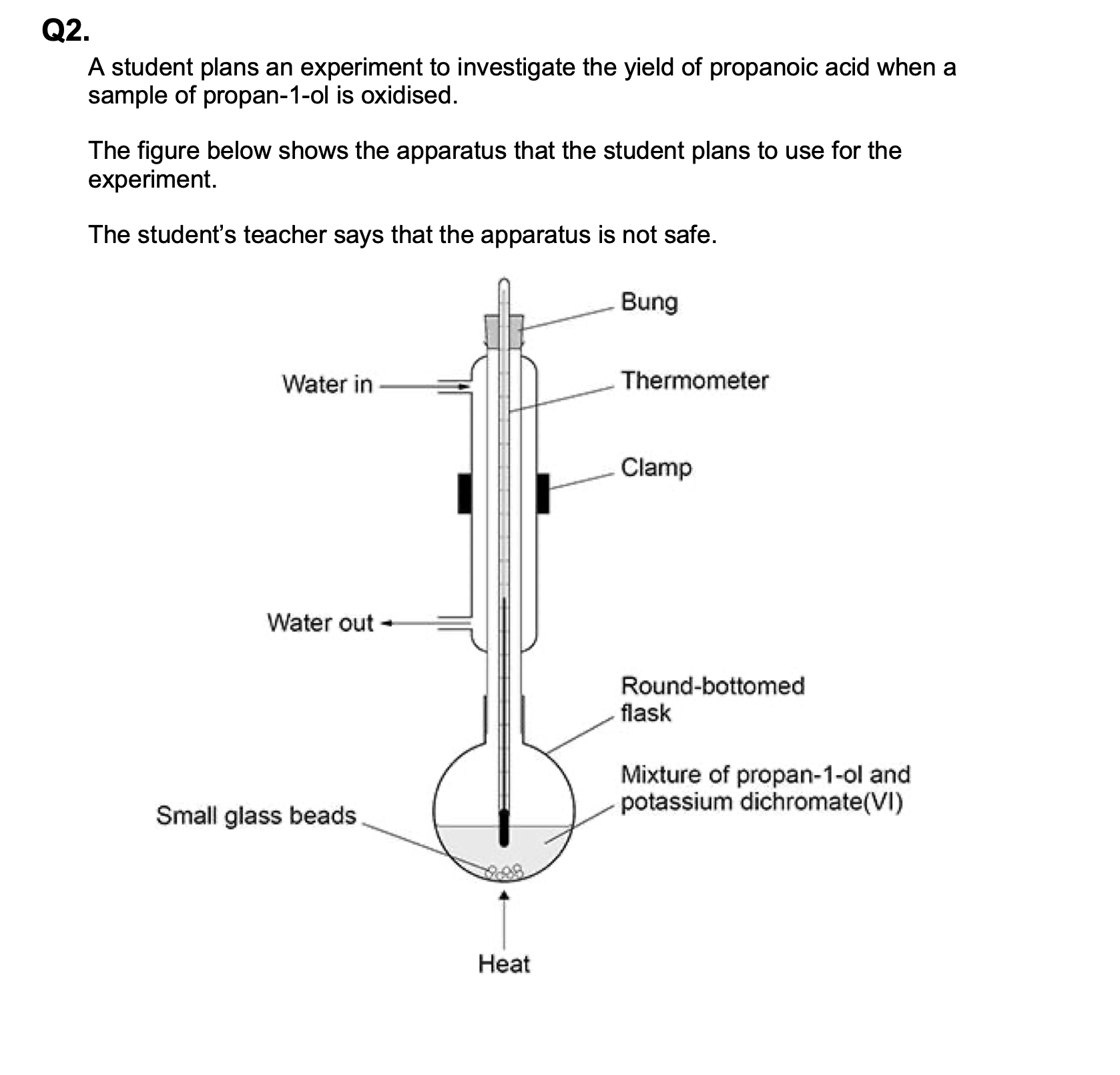
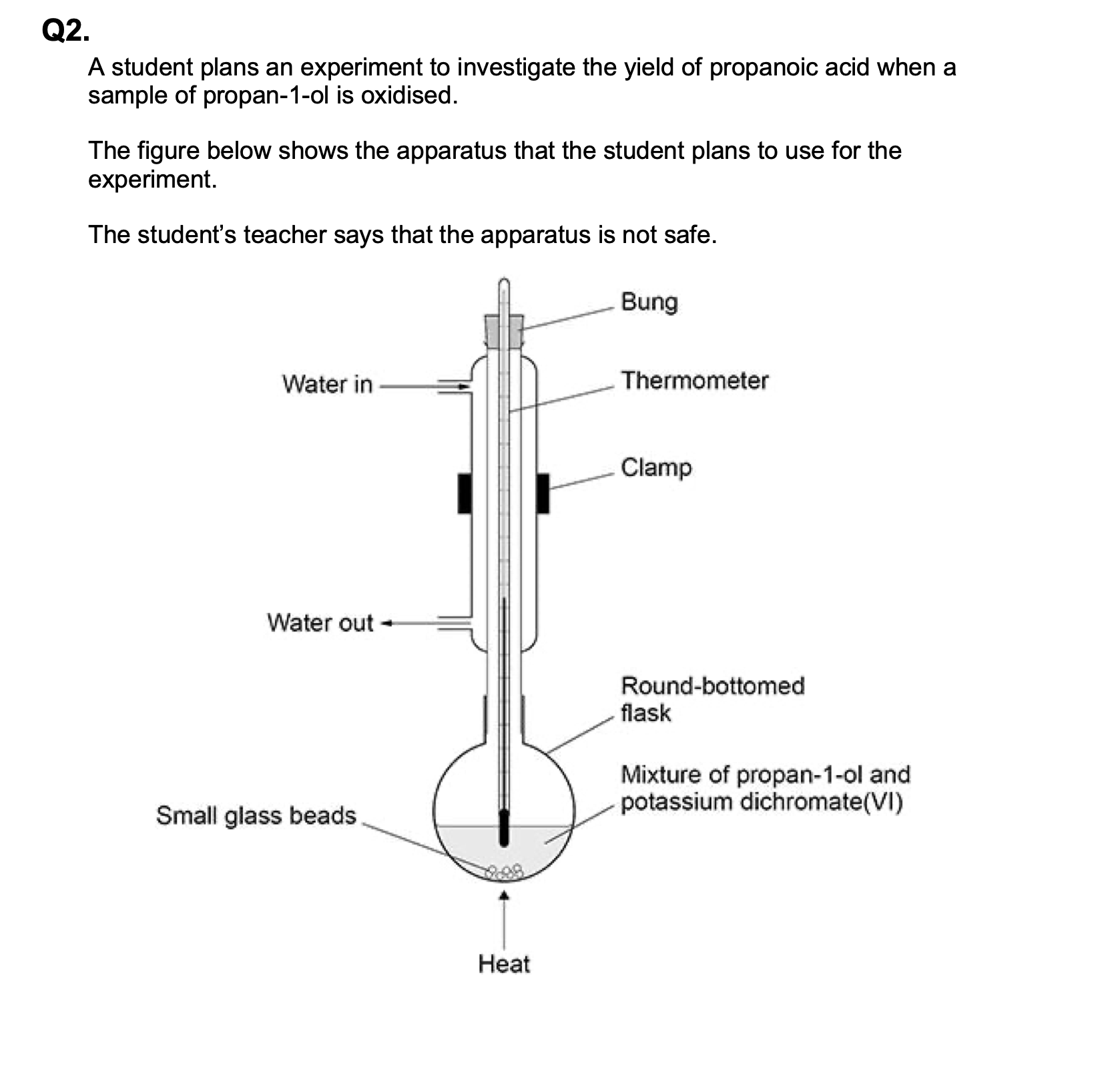
State two more mistakes in the way the apparatus is set up in above figure.
M1 direction of water flow through condenser
allow reference to water direction from answer to
(a)
M2 thermometer not needed
allow references to safety issue(s) if not given in
(a)
ignore reference to position of thermometer
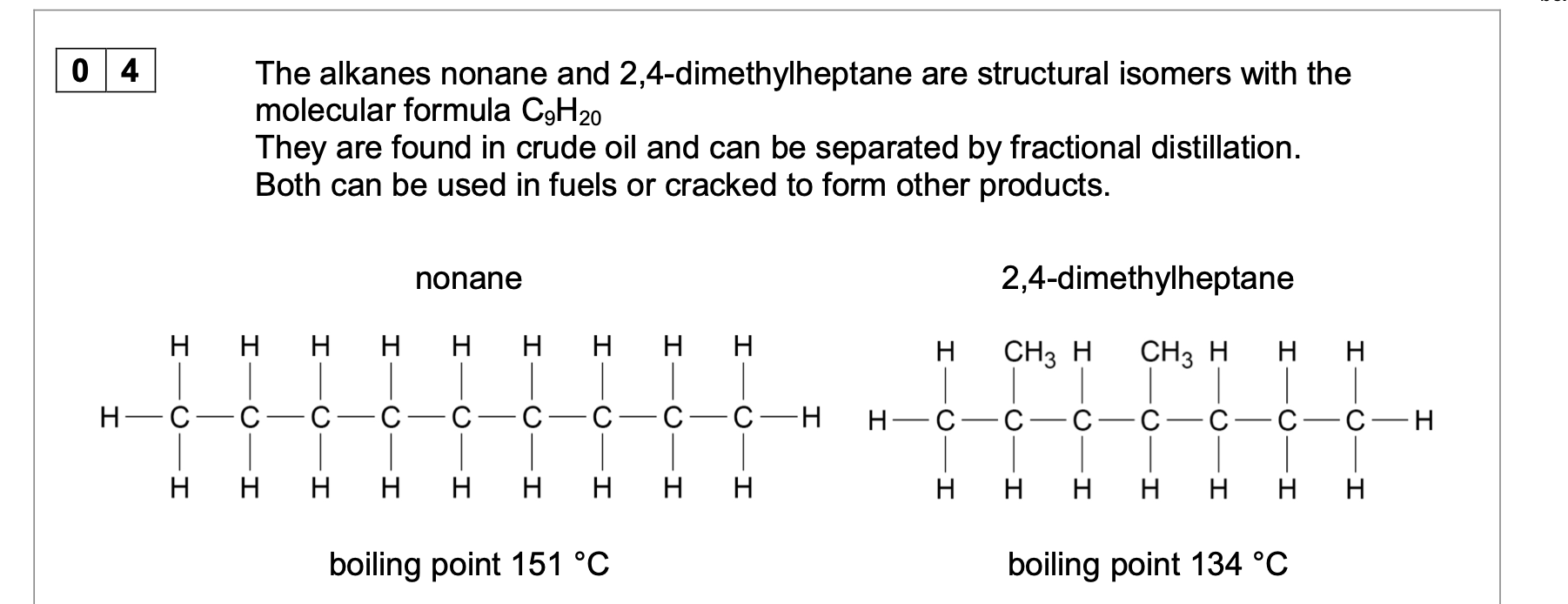
Explain why nonane has a higher boiling point than 2,4-dimethylheptane.

Structures
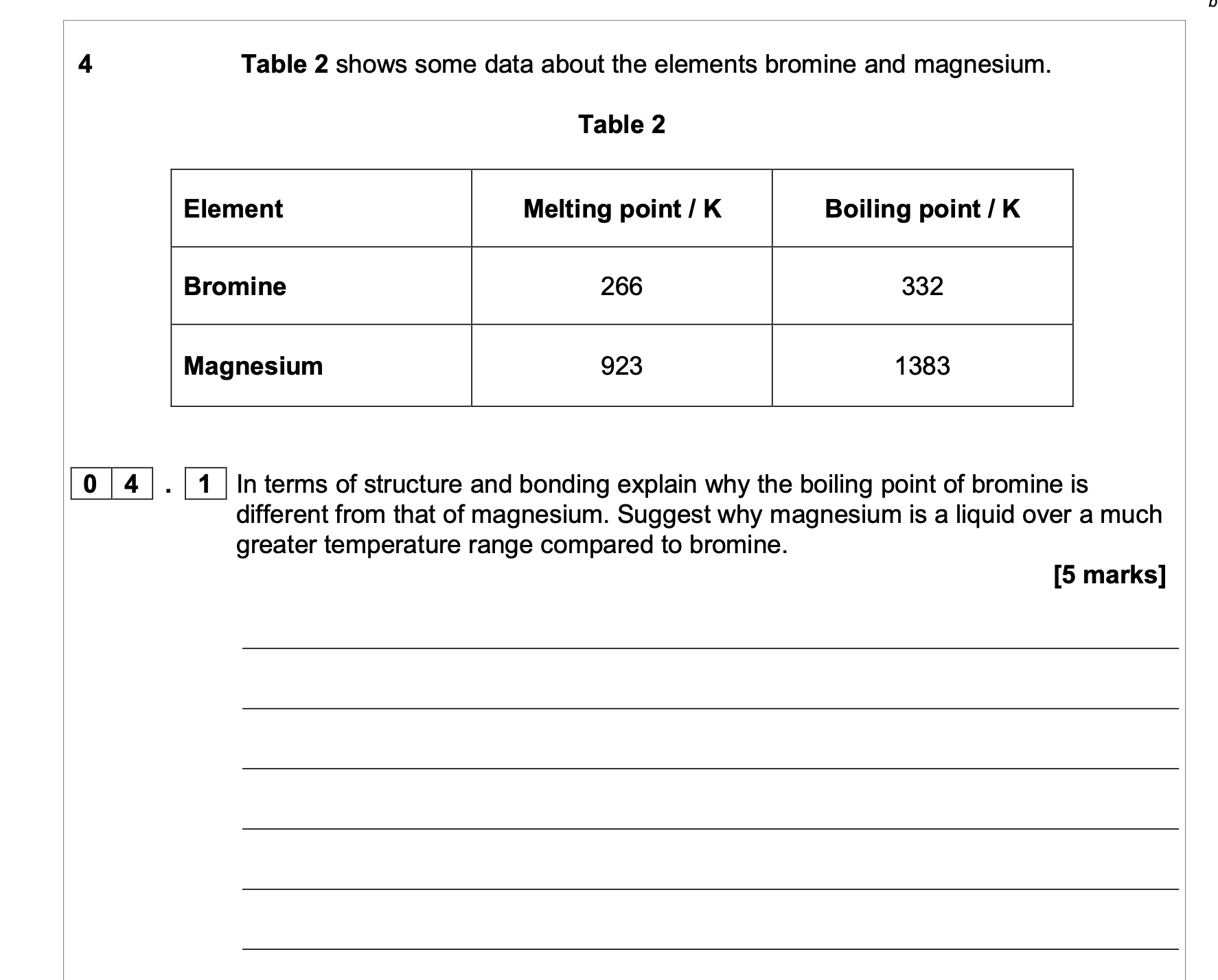
M1 Bromine is (simple) molecular / simple molecules
M2 Magnesium is metallic / consists of (positive) ions in a
(sea) of delocalised electrons
Strength
M3 Br2 has weak (van der Waals) forces between the
molecules / weak IMFs
M4 so more energy is needed to overcome the Stronger
(metallic) bonds or converse. The comparison could be direct
or implied.
Liquid range
M5 Mg has a much greater liquid range because forces of
attraction in liquid / molten metal are strong(er) OR converse
argument for Br2
1
1
Chemical Error penalties
If Br2 (covalent) bonds broken lose M3 and M4
If eg Mg molecules or Mg ionic bonds lose M2 and M4
1
1
1
Must refer to liquid range to score M5