Fielding Ch 14, 15, 17, 18; Glycocalyx and Lactate SOTAs
1/326
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
327 Terms
KDIGO Stage 1 Serum Creatinine
>0.3 mg/dL increase OR 1.5-1.9 x baseline
KDIGO Stage 1 Urine Output
<0.5 ml/kg/h for 6-12h
KDIGO Stage 2 Serum Creatinine
2.0-2.9 x baseline
KDIGO Stage 2 Urine Output
<0.5 ml/kg/h for 12 hours or more
KDIGO Stage 3 Serum Creatinine
>4.0 mg/dl OR 3.0 x baseline
KDIGO Stage 3 Urine Output
<0.3 ml/kg/h for 24h or more OR anuria for 12 hours or more
Common Risk Factors for the Development of AKI in Horses
Hypotension
Toxins
NSAIDs
Aminoglycosides
Oxytetracycline
Pigments (myoglobin and hemoglobin)
Cantharadin
Sepsis
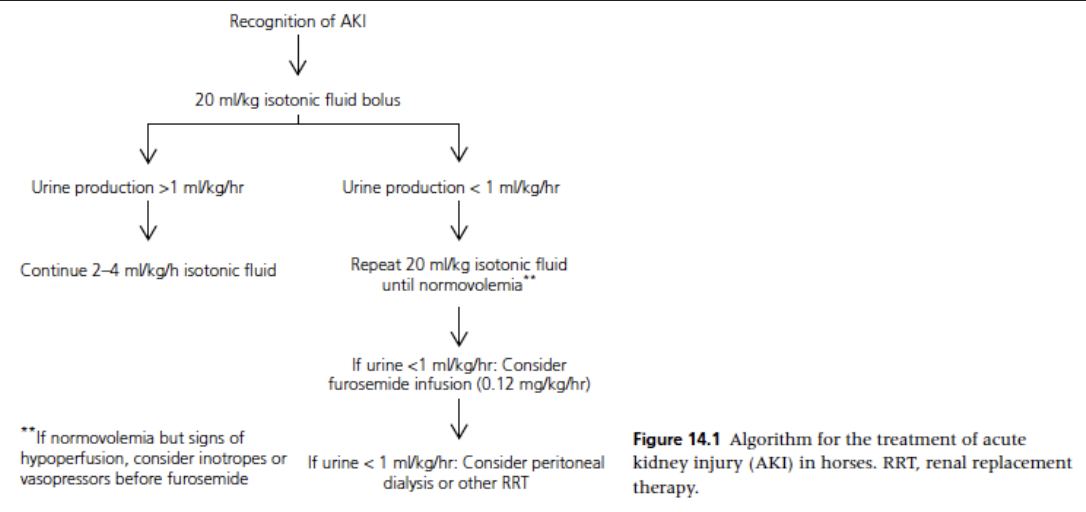
What is the most important treatment for patients with evidence of AKI and/or that are at risk for AKI?
IV fluid therapy
By restoring or maintaining perfusion to the kidney, further damage is mitigated and renal function may improve with time
In cases where the evidence of AKI is equivocal, moderate IV fluid administration has few contraindications and may prevent significant morbidity
In cases of anuria, IV fluids are still warranted, but they may carry a bigger risk if administration is excessive
What can improve decreased perfusion in AKI?
Inotropes and/or vasopressors that improve arterial blood pressure
What can you administer in patients with AKI that have adequate perfusion but diminished urine production?
Medications such as loop diuretics, mannitol, and dopamine
Isotonic Crystalloids for AKI
Isotonic crystalloid fluid such as LRS, a commercially available acetated fluid (Normosol-R, Plasma-Lyte A or Plasma-Lyte 148) or 0.9% saline may be an appropriate initial choice for fluid resuscitation in horses with AKI
Provide expansion of the extracellular fluid volume (more than a hypotonic fluid) and increased perfusion to the kidneys
Recent studies have shown benefit to lower chloride fluids and a "chloride restrictive" strategy
Hypertonic Saline for AKI
Hypertonic saline can be administered quickly and is relatively inexpensive compared to other crystalloids and colloids
Dose of 4 ml/kg of 7.2% sodium chloride can be used in place of the initial 20 ml/kg isotonic crystalloid dose for a horse with AKI
Compared to 0.9% saline, hypertonic saline decreased time to first urination and produced greater falls in PCV and total protein concentration in endurance horses
Hypertonic saline can produce more significant electrolyte abnormalities (specifically hyperchloremia) which may be more difficult to correct in a horse with renal insufficiency than one with normal function
In AKI induced by rhabdomyolysis, the potentially acidifying effects of hypertonic saline may not be optimal, as alkalinizing solutions have shown benefit in human studies with myoglobin-induced kidney injury
Sodium Bicarbonate for AKI
Horses tend to have a higher urine pH than humans so the benefits of sodium bicarbonate therapy may be even less clear
Potassium Supplementation for Horses with AKI
If urine production is diminished, additional potassium should be avoided
In documented hypokalemia, potassium supplementation should be considered but serum potassium concentrations should be monitored carefully
Calcium Supplementation for Horses with AKI
In cases of documented hypocalcemia, calcium supplementation should be considered
When clinical and laboratory evidence of sepsis is present, calcium supplementation should be avoided
Conclusion for Type of Fluids for AKI
Initiate fluid therapy for AKI using LRS or Normosol-R unless specific electrolyte derangements require a different type of fluid
Rate of Administration of Fluids for AKI
Initial 20 ml/kg bolus of an isotonic crystalloid (over 30-60 minutes) or a 4 ml/kg bolus of hypertonic saline (over 10 min) can be used for rehydration in horses with AKI
Begin with bolus unless signs of fluid overload are present
Following each 20 ml/kg bolus, clinical exam findings and other fluid balance variables - CVP and arterial blood pressure if available - should be reviewed
If urine output has begun or increased, the patient can be changed to a rate of 2-4 ml/kg/h with careful monitoring of continued urination
If significant urine is not being produced (>1-2 ml/kg/h) but signs of hypovolemia have resolved, additional medications to produce urine flow should be considered
If signs of fluid overload (edema, respiratory dysfunction) develop, fluid administration should be stopped until urine production develops
Ultrasound of the Urinary Bladder to Monitor Urine Production
Ultrasound of the urinary bladder may be a useful way to monitor urine production
A change in bladder size is one way to asses the production of urine if urination is not observed
In foals that may not be emptying their bladder, it is essential to document change in bladder size if a urinary catheter is not in place
Ideal Rate of Fluid Administration for Patients with AKI
Ideal rate of fluid administration for patients with AKI unknown
Due to association between fluid overload and mortality in patients with AKI a more conservative approach targeting a neutral or even slightly negative fluid balance once normothermia has been attained is advocated
Body weight measurements or "ins" and "outs" monitored to assess fluid balance
Conclusion for Fluid Rate for Fluids for AKI
Utilize 20 ml/kg crystalloid fluid boluses and reassess perfusion and hydration status after each is administered. Once urine production has been normalized, decrease to a rate of 2 ml/kg/h. If urine production is minimal (<1 ml/kg/h) despite adequate volume administration, consider medications to increase urine flow
Endpoint for Fluid Administration for AKI
Endpoint to fluid administration depends on the rate of urine production, persistence of risk factors, and the ability/willingness of the patient to drink
In horses that are maintaining a normal fluid balance (urine production matching fluid administration/intake) and where risk factors for AKI have been discontinued, IV fluids can often be stopped after 24-48 hours if renal values have normalized or even if they haven't if the patient is drinking enough to maintain renal perfusion
If azotemia persists despite therapy, IV fluids can continue until there is no longer a decrease in creatinine over a 48-72 hour period
Serum creatinine values may continue to decrease over 5-10 days or longer
Inotropes and Vasopressors for AKI
If perfusion is considered to be inadequate despite appropriate fluid therapy, then blood pressure can be improved with the use of dobutamine, norepinephrine, or similar medications
Medications to Increase Urine Production in AKI
Furosemide, dopamine, and mannitol
Aminophylline described as well
Many agree that these medications can increase the production of urine, there is considerable doubt as to whether they alter the long-term outcome of the case in human patients
Furosemide should be considered as a treatment for fluid overload and as a means to attempt to increase urine production in anuric/oliguric horses, but not necessarily as a treatment for AKI
Loop Diuretics for AKI
Can theoretically reduce renal tubular oxygen demand by decreasing the energy requirements of the cells in the thick ascending limb of the loop of Henle
Furosemide produces an increase in urine flow, but it does not appear significantly to alter renal outcome
Furosemide is the most commonly used loop diuretic in horses and has a significant role in the management of fluid overload and electrolyte derangements
Can be given both as a bolus administration (0.1-1.0 mg/kg) or as a CRI (0.12 mg/kg/h)
In horses with anuria or fluid overload, furosemide is an essential part of the treatment plan
Unlikely to improve renal damage, but may give more time to address ongoing risk factors and electrolyte derangements and possibly time for the kidneys to heal
Dopamine for AKI
Low dose dopamine (0.5-3 ug/kg/min) has been shown to increase renal blood flow, promote natriuresis, and increase urine flow in the first 24 hours of treatment in humans
No evidence to suggest that it alters the outcome in AKI
If fluid therapy and furosemide have not increased urine production in a horse with AKI and fluid overload, low-dose dopamine is a reasonable treatment to try
Mannitol for AKI
Has been shown to increase urine flow, which can have benefits in the management of fluid overload associated with AKI
In cases where furosemide and dopamine are not able to increase urine production, mannitol may be considered
Dose of 1-2 mg/kg/min after a slow IV bolus of 0.25-0.5 g/kg
A total dose of 1 g/kg should not be exceeded if urine production has not been initiated by that point
If fluid overload is present, mannitol should not be bolused as it can further expand plasma volume, but the low CRI dose can be tried for a few hours as long as CVP and clinical status are monitored closely
Aminophylline for AKI
No adverse effects in studies but more research needed to evaluate the use in people and horses
Renal Replacement Therapies (RRTs) for AKI
Includes hemodialysis, peritoneal dialysis, hemofiltration, and kidney transplant
Peritoneal dialysis most commonly described in clinical cases of renal failure in horses
In clinical cases of AKI that develop anuria, renal replacement therapies may be an option for some horses
Recommendations: Horses with AKI that cannot be managed with intravenous fluids and medications to increase urine production (furosemide, dopamine, etc) should be considered candidates for RRT. At this time peritoneal dialysis may be the most practical for equine veterinary patients
Foals and AKI
Sick newborn foals often present to equine hospitals with GI, neurologic, and renal dysfunction as a triad of problems possible associated with a period of hypoxia surrounding parturition
Sepsis is also a common abnormality in newborn foals that has been associated with AKI in many species
Treatment options are similar to adults but neonatal foals have different fluid balance physiology and may be more prone to fluid overload compared to adults
Spurious Hypercreatinemia
Foals with increased serum creatinine at birth that rapidly declines over the first 24-48 hours
Chronic Kidney Disease
Fluid therapy is likely to be far more important in cases of AKI than CKD
IVF should be carefully considered for patients with CKS and a conservative fluid rate of administration is often indicated
Horses are likely prone to fluid overload when CKD is present
Hypercalcemia is common in horses with CKS and additional supplementation should be avoided
Causes of Intravascular Volume Deficits in Horses with Liver Failure
Lack of fluid intake
Decreased vascular tone and/or endothelial dysfunction
Decrease in vascular tone may be the most important factor
Increased urinary loss
May in some cases be associated with a decrease in hepatic urea synthesis and a low serum BUN
Results in a decrease in urea-associated renal interstitial osmolality, which would decrease the effectiveness of vasopressin on renal water resorption
Hypertonic Saline for Resuscitation in Horses with Liver Failure
Hypertonic saline (7.5%, 4 ml/kg) can be administered in adult horses with liver failure if there is clinical or measurable evidence of severe hypotension and abnormally low cardiac preload
Possible disadvantages would be the large-volume urination that usually occurs following treatment causing potassium loss in the urine (kaliuresis)
Crystalloids for Resuscitation in Horses with Liver Failure
An alternative to the administration of hypertonic saline would be to administer in the first hours of therapy 50 ml/kg of a balanced crystalloid with 50 g of dextrose and 20 mEq of KCl added
Provides a volume of fluid nearly equal to a normal intravascular plasma volume
Ideally a crystalloid with an acetate buffer should be used rather than one with a lactate buffer
Monitoring for Resuscitation for Horses in Liver Failure
Persistent elevations in PCV in spite of favorable findings for other cardiovascular parameters is not unusual in horses with acute liver failure, chronic liver disease that is severe, or hepatic neoplasia
A decline in plasma lactate would be an indication of improved perfusion and is likely a favorable prognostic finding
Since the liver is a major organ for lactate metabolism and LRS may sometimes be given for resuscitation, it is possible that tissue perfusion has been improved but might not be reflected by a comparative decline in plasma lactate
Fluids Following Resuscitation for Horses in Liver Failure
Following initial successful resuscitation, approximately twice maintenance fluid rate (120 ml/kg/day) with an acetate buffered balanced electrolyte solution is indicated
Dextrose (generally 50-100 g/L) and KCl (generally 20-240 mEq/L) should be continued depending upon the clinical condition of the horse, laboratory chemistry monitoring, and oral consumption of fluids
Factors to Avoid to Prevent Cerebral Edema in Horses with Hepatic Encephalopathy
Overhydration
Respiratory acidosis or alkalosis
Serum sodium derangements
Hypokalemia
Serum calcium derangements
Hypo-oncotic states
Low head position
Excessive sedation
Hypovolemia/hypotension
Hyperthermia
Sedatives in Horses with Hepatic Encephalopathy
Sedatives may decrease ventilation, increase PCO2, and result in vasodilation within the brain which could contribute to cerebral edema
Decreasing Production of Ammonia/Ammonium in Horses with Hepatic Encephalopathy
All efforts should be made to decrease the production of ammonia/ammonium, prevent their diffusion into the central nervous system, and increase their elimination
Maintaining adequate urine production and normal plasma K+ concentration will enhance renal ammonia/ammonium excretion
Should colloids be used in liver failure?
Colloid therapy will substantially increase the cost of treatment without proven benefit
If colloids are used, fresh-frozen plasma (FFP) or 25% human albumin may have some therapeutic advantages
25% Human Albumin for Liver Failure
Plasma albumin treatment may increase plasma oncotic pressure, which will prolong and enhance the plasma volume restoration effect of crystalloid therapy
Albumin may provide an antioxidant effect and potentially bind endogenous toxins that may be involved in the pathophysiology of HE
Although the liver is the sole producer of albumin, severe hypoalbuminemia is rare in horses with either acute or chronic liver failure
May be related to the longer half-life of equine albumin (20 days) or the ability of the equine liver to produce albumin in spite of tremendous loss of function
Administration of 25% human albumin would have a more dramatic effect on plasma oncotic pressure than fresh frozen equine plasma but is rarely used in horses
Would be a foreign antigen and doesn't provide regulating coagulation/inflammatory factors as does equine FFP
Fresh Frozen Plasma for Liver Failure
FFP provides many proteins other than albumin that may be decreased with severe liver disease
Includes the non-endothelial derived clotting factors (II, V, VII, IX, X, XI, XIII) and other regulatory proteins such as protein C, protein S, antithrombin III, and fibronectin
Prothrombin time (PT) can very quickly be prolonged following LF, naturally occurring bleeding abnormalities are uncommon in horses with liver failure
Liver biopsy rarely causes notable hemorrhage in horses with LF in spite of prolonged clotting times, possibly because of normal platelet counts in most horses with LF
Horses with LF that require nasogastric intubation or those in need of surgery could be given FFP in an attempt to improve clotting function prior to the procedure
Large amounts of plasma (10-15 ml/kg) may be needed to return PT and PTT to normal ranges
Smaller amounts (2-8 ml/kg) would be less expensive, decrease the chance of volume overload, and may reduce the possibility of hemorrhage even if clotting function tests remain outside the normal range
If a whole blood transfusion is needed, it should be freshly collected because even with proper collection and storage for a short time (days), high ammonia concentrations can occur in the transfused blood and this should be avoided
Hetastarch in Horses with Liver Failure
Hetastarch is best avoided in horses with LF as it may further prolong clotting times and its storage in hepatocytes and Kupffer cells may cause further deterioration in hepatic function
Ventral Edema with Equine Hepatic Lipidosis
Ascites caused by LF is rarely reported in horses
Equine hepatic lipidosis sometimes causes marked ventral edema
May be the result of an acute increase in hydrostatic pressure in the subcutaneous abdominal veins
Increased hydrostatic pressure may be caused by the acute need for these veins to carry a increased blood volume from the abdomen to the heart; this demand for alternate venous return may be due to the resistance to portal flow caused by the rapidly enlarging liver
Sodium Abnormalities in Hepatic Failure
Possible that a part of the hyponatremia observed in horses with hepatic lipidosis may be spurious
Hyperlipemia may cause a false decrease in the sodium
Ponies and miniature horses with hepatic lipidosis, hyperlipemia, and ventral edema may best be treated with a lower sodium fluid such as 0.45% NaCl + 2.5% dextrose + 20-40 mEq/L of KCl for maintenance purposes and 0.1 U of insulin/kg while monitoring both glucose and potassium concentrations
Potassium Abnormalities in Horses with Hepatic Failure
Potassium homeostasis may be more important than sodium abnormalities in most horses with LF
When horses become anorexic, as in LF, today body potassium and extracellular potassium may be quickly depleted
Decrease in effective plasma volume as expected with LF would likely increase plasma aldosterone concentrations, which may further decrease plasma potassium through enhanced loss in the urine
Plasma potassium concentrations may be variable depending upon renal function, acid-base abnormalities, and plasma glucose concentrations
Horses with Theiler's disease and those with end stage liver disease may occasionally develop intravascular hemolysis, which may increase plasma potassium concentration
Fluid therapy used to correct and maintain an adequate circulating intravascular volume will in most cases cause a net loss of potassium (even when added to the fluids) because of increased urine production and kaliuresis
Proposed that potassium deficiency is a pathophysiologic factor in HE
May be a result of a relationship between potassium and ammonia metabolism, hypokalemia increases plasma ammonia and ammonium concentrations
Potassium Supplementation in Liver Failure
In horses with LF that are producing urine following rehydration, potassium should be supplemented at 20-40 mEq/L
Abnormalities in Glucose Homeostasis with Liver Failure
Glucose is the primary organ responsible for glucose production
Glucose support should be considered in the fluid therapy plan for all patients with LF
Most adult horses with LF are normoglycemic or hyperglycemic
Hyperglycemia may be at least partially the result of hyperammonemia
High NH3 enhances the release of glucagon and causes insulin resistance
Glucose Supplementation in Liver Failure
Glucose as a 5% solution should initially be added to the crystalloid fluids provided to horses with LF but may need to be adjusted
For marked hypoglycemia as is commonly seen in foals with LF, the initial intravenous fluid dextrose concentration should be 10%
Insulin should be administered to equines with hyperlipemia and blood glucose concentrations above 180 mg/dL
Glucose and/or insulin administration will decrease plasma potassium concentrations
Intravenous Nutritional Support for Hepatic Failure
Glucose is the predominant intravenously administered nutritional support for horses with LF
Forced enteral feeding is preferred, but partial parenteral nutrition using amino acids and glucose can be life saving in those that cannot be fed enterally
Form of protein provided in parenteral nutrition or enteral support is controversial for patients with liver disease
Products high in branched chain amino acids are generally preferred
The less expensive standard protein solutions of crystalline amino acids might be equally useful in ponies, donkeys, and miniature horses with hyperlipemia that cannot be fed enterally
Most horses with LF have a negative energy balance and any protein supplements will be primarily "burned" for calories
Horses with LF should not be maintained for more than 5 days on dextrose alone without provision of some protein, as this may cause hepatic lipidosis
All horses with LF should be administered multi-B vitamins to support cellular metabolism in the liver and other organs
What form of protein is preferred in horses with hepatic failure?
Products high in branched chain amino acids
What is the liver involved in the production and metabolism of?
Metabolism of organic acid anions (i.e. lactate)
Metabolism of ammonia
Production of albumin
What is the predominant acid base disturbance in horses with liver failure?
Metabolic acidosis and acidemia
Respiratory Compensation for Metabolic Acidosis in Liver Failure
May be partial compensatory respiratory alkalosis in horses with HE, but these horses may be more prone to respiratory acidosis due to hypoventilation
What causes metabolic acidosis with liver failure?
Metabolic acidosis in horses with acute liver failure is likely a result of hypoperfusion, increased anaerobic metabolism, and production of lactic acid as well as a decrease in the clearance of lactate by the failing liver and as a response to hyperammonemia
Liver is responsible for at least 50% of lactate metabolism
Lactic Acidosis in Horses in Liver Failure
Lactic acidosis in horses with LF should be treated as it may cause impaired myocardial contractility and decrease vascular response to catecholamines
Treatment should focus on improving cardiac output and improving perfusion of all tissues including the liver
Best accomplished by administration of non-lactate containing crystalloids and correction of electrolyte abnormalities that may be undermining normal cardiac and endothelial cell function
Also normalize glucose concentrations and inhibit inflammatory cytokines and prostanoids
Using Sodium Bicarbonate to Treat Horses with Metabolic Acidosis Due to Liver Failure
Sodium bicarbonate should not be used in the treatment of horses with metabolic acidosis and LF
It may rapidly increase the plasma ammonia concentration by shifting the ammonium:ammonia equilibration toward gaseous ammonia (NH3) which readily crosses an intact blood-brain barrier
May also lower plasma ionized calcium and potassium concentrations and even increase respiratory acidosis in horses with HE that are hypoventilating
What is heart failure?
A clinical syndrome in which cardiac dysfunction leads to the inability of the heart to pump adequate blood forward
What are the three mechanisms that can cause heart failure?
Volume overload
Pressure overload
Primary myocardial disease
What is the most common mechanism of heart failure in horses?
Volume overload
What are the hemodynamic consequences of heart failure?
Increased ventricular filling pressure, which leads to venous congestion
Reduced cardiac output
What are the most common causes of heart failure in horses?
Congenital cardiac defects leading to left-to right shunting (VSDs leading to left-sided heart failure)
Chronic degenerative valve disease in older horses (leading to left-sided, right-sided, or bilateral heart failure)
Pericardial disease resulting in cardiac tamponade (leading to signs of right-sided heart failure)
Clinical Signs of Left-Sided Congestive Heart Failure
Pulmonary edema -> cough, tachypnea, dyspnea, hemoptysis, frothy nasal discharge
Clinical Signs of Left-Sided Output Heart Failure
Systemic hypoperfusion -> exercise intolerance, weakness, lethargy, cool extremities, and hypothermia
Clinical Signs of Right-Sided Congestive Heart Failure
Subcutaneous edema, peripheral venous distension, hepatomegaly, ascites -> ventral edema, abdominal distension, and peripheral venous distension
Clinical Signs of Right-Sided Output Heart Failure
Pulmonary hypoperfusion (can lead to left-sided output failure) -> exercise intolerance, weakness, lethargy
Bilateral or Biventricular Heart Failure in Horses
Left-sided congestive heart failure can lead secondarily to right-sided congestive heart failure so horses can present with signs of bilateral or biventricular heart failure
Postulated to be due to a propensity for pulmonary vascular remodeling and vasoconstriction with increased left-sided filling pressure
What are the neurohormonal compensatory mechanisms that temporarily preserve organ function once heart disease results in cardiac output?
Sympathetic nervous system
RAAS
Vasopressinergic system
Endothelin
Kallikrein-kinnogen-kinin system
Natriuretic peptide system
Compensatory Action of the Sympathetic Nervous System in Heart Failure
Tachycardia, increased inotropy, and vasoconstriction
Compensatory Action of the RAAS in Heart Failure
Sodium and water retention and vasoconstriction
Compensatory Action of the Vasopressinergic System in Heart Failure
Water retention and vasoconstriction
Compensatory Action of Endothelin in Heart Failure
Vasoconstriction
Compensatory Action of the Kallikrein-Kininogen-Kinin System in Heart Failure
Vasodilation
Compensatory Action of the Natriuretic Peptide System in Heart Failure
Diuresis
Fluid Balance in Heart Failure
Paradox is the simultaneous arterial hypovolemia in the face of total ECF overload (with venous hypervolemia)
Decrease in effective forward arterial flow as cardiac output declines with heart failure is sensed by baroreceptors in the carotid sinus, aortic arch, and renal afferent arteriole
Sodium and water retention and vasoconstriction result from activation of the sympathetic nervous system, the RAAS, and the vasopressinergic system
Imbalance between effective forward flow and total body fluid volume makes treating chronic heart failure a challenge
What are the most important consequences of heart failure?
Most important consequence of heart failure is congestion leading to pulmonary edema (left-sided) and subcutaneous edema and ascites (right-sided)
Secondary to increased hydrostatic pressure in the pulmonary and systemic capillary systems, respectively
Decreasing hydrostatic pressure by decreasing blood volume is essential
Treatment of Acute Congestive Heart Failure
Fluid administration is contraindicated in cases of congestive heart failure
Immediate treatment for acute congestive heart failure should include diuretics and oxygen supplementation
Treatment of Chronic Heart Failure
Treatment of chronic heart failure should include diuretics and (ideally) ACE inhibitors
Loop Diuretics in Heart Failure
Furosemide is the diuretic of choice in humans and animals
For a given level of natriuresis, furosemide produces superior diuresis when compared with other diuretics
Furosemide will work despite the presence of renal impairment, which is common with heart failure
There is increasing response to increasing doses (a "high ceiling" diuretic)
MOA of Loop Diuretics
Inhibits the luminal Na+/K+/2Cl- cotransporter in the thick ascending loop of Henle, reducing the reabsorption of these electrolytes (and therefore water), resulting in effective diuresis
What does IV administration of furosemide in horses result in?
Decrease in blood volume, ECF volume, left ventricular filling pressure, and pulmonary capillary pressure
Furosemide Administration for Heart Failure in Horses
Initial emergency dose in horses is 1-2 mg/kg
Can be followed by additional IV boluses as early as 30-60 minutes or as late as 6-8 hours after the first dose
Furosemide infusions (0.12 mg/kg/h) have been shown to result in a greater and more consistent decrease in plasma volume with fewer adverse effects on renal function than equivalent IV bolus dosing
No standard total daily dose, the dose that should be used is the minimum amount needed to clear the pulmonary edema
Furosemide for Chronic Heart Failure
Furosemide is also the cornerstone of treatment for chronic heart failure
Suggested dose for chronic heart failure management is 1-2 mg/kg 2-3 times daily
Furosemide is poorly and variably absorbed when given orally so shouldn't be administered PO
Loop Diuretics other than Furosemide
Other loop diuretics (torsemide, bumetanide) can be used in refractory cases of heart failure
Adverse Consequences of Furosemide
Activates deleterious neurohormonal systems (sympathetic nervous system, RAAS, and vasopressinergic system), induces hypovolemia and dehydration, and creates electrolyte and acid-base imbalances (hyponatremia, hypochloremia, hypokalemia, and metabolic alkalosis)
Activation of the neurohormonal systems increases sodium and water retention and causes vasoconstriction, both undesirable in heart failure
Also has direct adverse fibrotic remodeling effects on the myocardium via angiotensin II and aldosterone, worsening cardiac function
Furosemide decreases blood volume so decreased tissue perfusion and cellular dehydration can result
Can cause hypokalemia and hyponatremia
Adverse Consequences of Furosemide - Adverse Fibrotic Remodeling Effect on the Myocardium
Administer furosemide with a medication to blunt the RAAS
Adverse Consequences of Furosemide - Decreased Blood Volume Leading to Decrease in Tissue Perfusion
Renal function may be jeopardized by reducing renal blood flow
Reduction in dose and discontinuation of ACE inhibitors and NSAIDs should be considered in patients developing renal failure
Adverse Consequences of Furosemide - Hypokalemia and Hyponatremia
Hypokalemia can be ameliorated by increasing potassium ingestion
Hyponatremia is a consequence both of heart failure itself and of chronic furosemide use
Release of AVP allows for solute free absorption which more readily contributes to hyponatremia than other mechanisms of water retention that rely on sodium retention
AVP release is stimulated by two mechanisms during heart failure
A decrease in arterial blood pressure
Angiotensin II
Hyponatremia should not be corrected with sodium supplementation
Thiazide Diuretics for Heart Failure
Hydrochlorothiazide inhibits the NaCl cotransporter in the distal convoluted tubule, reducing the reabsorption of sodium and chloride (and therefore water)
Because much of the filtered sodium has already been reabsorbed by the time it reaches the distal convoluted tubule, thiazide diuretics are less effective than the loop diuretics
Can cause serious hyponatremia and hypokalemia so if combined with furosemide it should be initiated at a low starting dose (0.5 mg/kg PO q24h)
Aldosterone Antagonists in Heart Failure
Spironolactone works by competitively inhibiting aldosterone in the late distal convoluted tubule, reducing sodium reabsorption (and therefore water) and enhancing potassium reabsorption
Because much of the sodium has already been absorbed by the time the filtrate reaches the distal convoluted tubule, the aldosterone antagonists are less effective diuretics than loop diuretics
Why are aldosterone antagonists commonly used as the second diuretic paired with furosemide in small animals?
The potassium-sparing effect can blunt the hypokalemia that can occur with furosemide use
Aldosterone antagonists have been shown to improve morbidity and mortality for people and dogs in heart failure despite being weak diuretics
Currently expensive to be used in horses with heart failure
ACE Inhibitors in Heart Failure
Ideally, in chronic heart failure cases, furosemide should be paired with ACE inhibition
As blood volume decreases following a dose of furosemide, the body's natural response is activation of RAAS
RAAS activation counteracts the previous dose of furosemide by increasing blood volume until the next dose of furosemide is given
ACE inhibitors decrease production of angiotensin II and therefore aldosterone
Which ACE inhibitor should be used in horses?
Most commonly used ACE inhibitor in small animal cardiology (enalapril) has been proven to be fairly ineffective in horses when given at the same dose
In horses, benazepril (0.5 mg/kg once daily) is the most effective ACE inhibitor, results in peak reduction of serum ACE activity of 87%
What is an adverse consequence of ACE inhibitors?
Renal impairment is an adverse consequence of ACE inhibition, as blocking the production of angiotensin II alters intrarenal hemodynamics
Blocks synthesis of protective prostaglandins which can lead to renal ischemia, a decline in glomerular filtration pressure, and acute renal failure
Pathophysiology of Cardiac Tamponade
Occurs when intrapericardial pressure increases and then exceeds the normal diastolic filling pressure within the cardiac chambers
Results in equilibration of the intrapericardial and cardiac filling pressures (i.e. the intrapericardial pressure dictates the cardiac filling pressure)
Transmural pressure gradient decreases, which affects right heart filling before left heart filling since the right heart normally operates at a lower transmural filling pressure
Pericardial fluid accumulates and leads to collapse of the cardiac chambers, decreased cardiac filling during diastole, and therefore decreased cardiac output during systole
What are the factors that development of cardiac tamponade depends on?
Volume of the pericardial fluid
Rate at which the fluid accumulates
Properties of the pericardium itself (compliance)
Clinical Signs of Cardiac Tamponade
Clinical signs are similar to those of right-sided congestive heart failure - tachycardia, peripheral venous congestion, jugular pulsation, subcutaneous edema, ascites, and hepatomegaly
Other signs of cardiac tamponade are non-specific (fever, anorexia, lethargy, weight loss, colic, tachypnea) and specific (tachycardia, quiet heart sounds, pericardial friction rubs, weak arterial pulses, and pulsus peradoxus)
Causes of Pericardial Effusions
Pericardial effusions have been associated with viral and bacterial infections (EHV-1, EHV-2, influenza virus, Streptococcus spp., Actinobacillus equuli, Pseudomonas spp, Pasteurella multocida, Staphylococcus aureus, Acinetobacter spp, Escherichia coli, Enterococcus fecalis, Corynebacterium pyogenes, Mycoplasma felis, Propionibacterium acnes, Corynebacterium pseudotuberculosis, Clostridium spp), immune-mediated diseases such as eosinophilic pericarditis, neoplasia, trauma, or through contagious spread of nearby infectious or inflammatory processes
Many cases are considered idiopathic
Neurohormonal Compensation in Cardiac Tamponade
As diastolic filling and cardiac output decline, the resulting arterial hypovolemia will activate the neurohormonal systems that are activated in heart failure
Unlike in heart failure, the water retention and vasoconstriction is beneficial because preload is the only factor that can combat the interapericardial pressure increase
Increasing afterload is not detrimental because systolic function is usually normal
Fluid Balance in Cardiac Tamponade
There is the same paradox as in heart failure with arterial hypovolemia in the face of systemic venous hypervolemia
Preload must be increased to drive cardiac filling