Section 1 Lectures 4-6
5.0(2)
5.0(2)
Card Sorting
1/55
Earn XP
Description and Tags
Proteins, Nucleic Acids, Energy, ATP, Enzymes, Sugars, Lipids
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
56 Terms
1
New cards
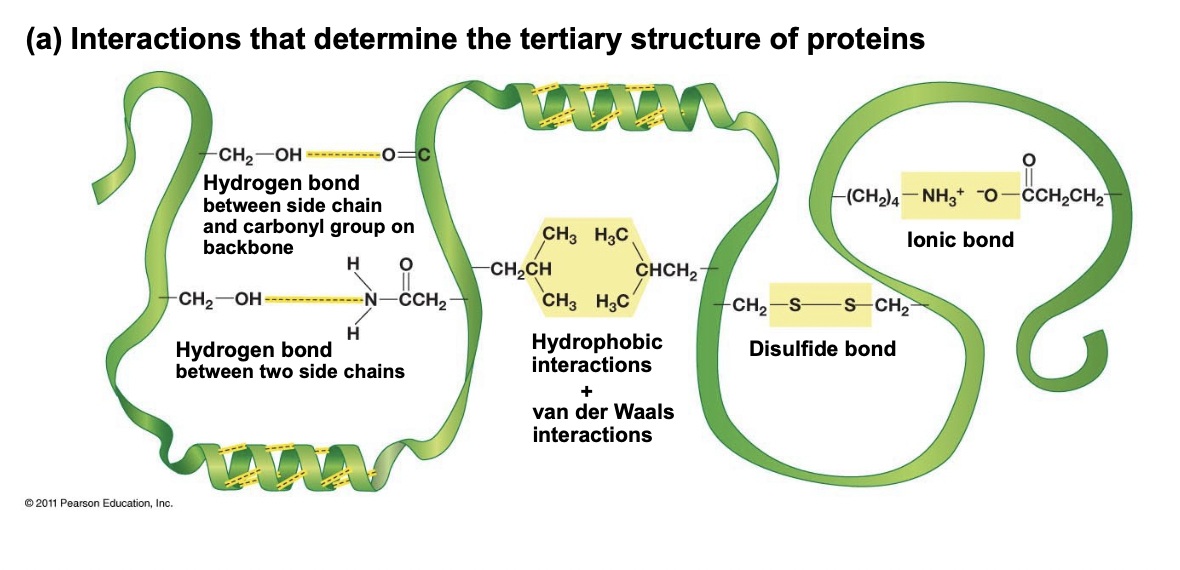
Tertiary Structure
* Overall 3D shape of polypeptide, final folding
* Occurs due to side chain interactions
* Interactions like: H-bonds between 2 chains, hydrophobic and van Der Waals interactions etc.
* Occurs due to side chain interactions
* Interactions like: H-bonds between 2 chains, hydrophobic and van Der Waals interactions etc.
2
New cards
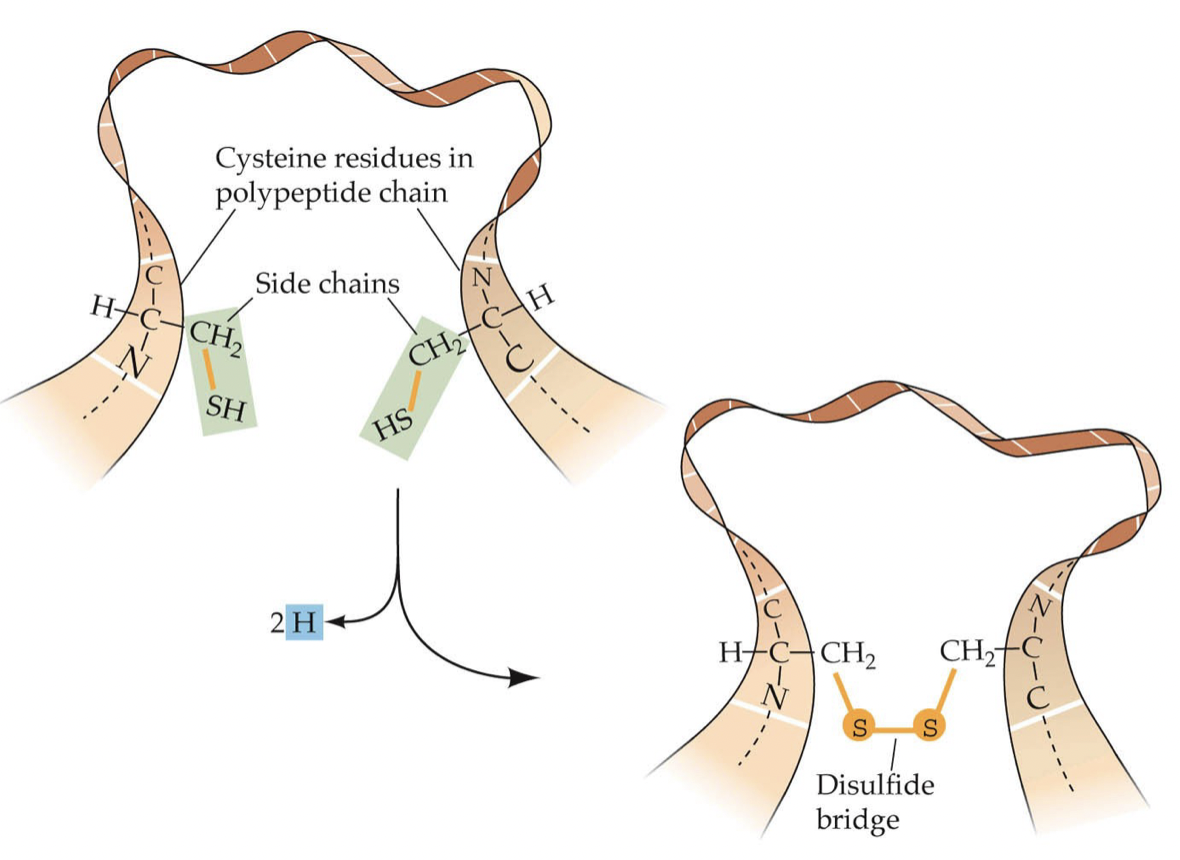
Disulfide Bridge
* 2 Cysteine side chains are close (in space, not chain) so the two S atoms bond
* Bond via redox reaction
* Bond via redox reaction
3
New cards
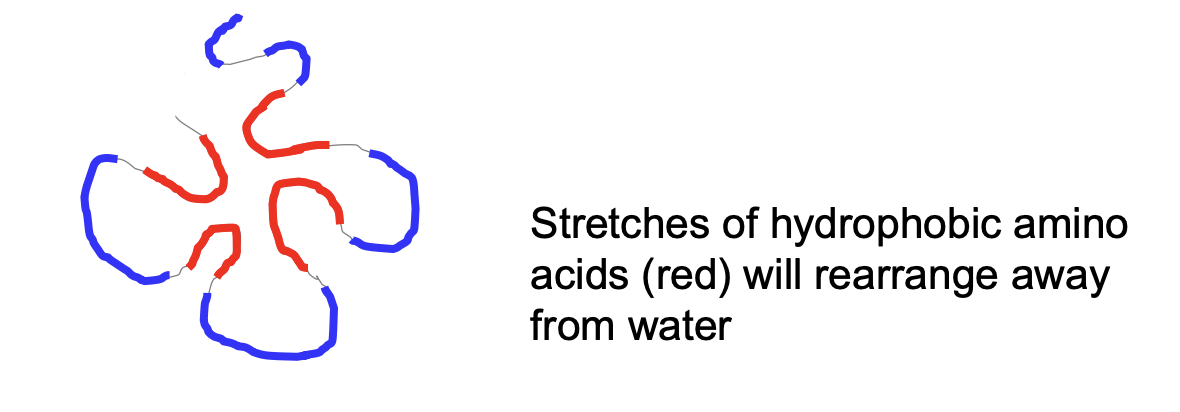
Rearranging of Hydrophobic Amino Acids
* Rearrange themselves away from water
* Hydrophobic amino acids face inside and hydrophilic will face outside
* Hydrophobic amino acids face inside and hydrophilic will face outside
4
New cards
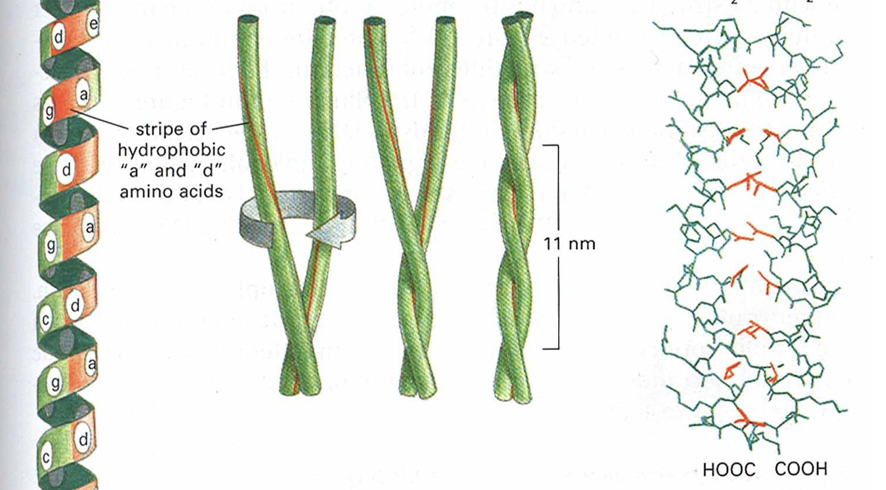
Coiled Coils
* Arise when two alpha helices have hydrophobic amino acids at every 4th position
* 2 helices wrap around each other to avoid water
* Very stable structure that shows up in hair and feathers (keratin protein)
* If 2 helices from the same structure, will have a loop, tertiary structure
* If 2 helices from different structures, no loop, quaternary structure
* 2 helices wrap around each other to avoid water
* Very stable structure that shows up in hair and feathers (keratin protein)
* If 2 helices from the same structure, will have a loop, tertiary structure
* If 2 helices from different structures, no loop, quaternary structure
5
New cards
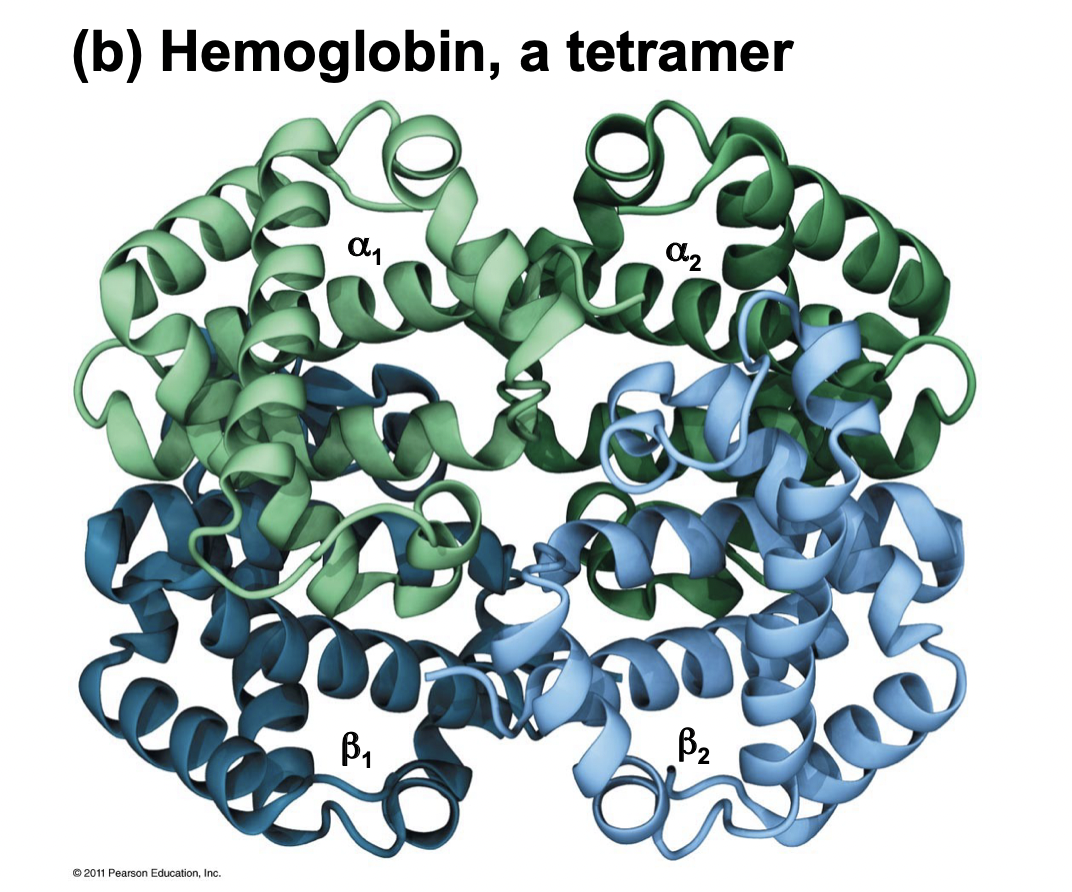
Quaternary Structure
* Occurs when tertiary structures still do not have function
* Final step of protein folding
* Several polypeptides form a large protein complex
* Ex. hemoglobin is made of 4
* Final step of protein folding
* Several polypeptides form a large protein complex
* Ex. hemoglobin is made of 4
6
New cards
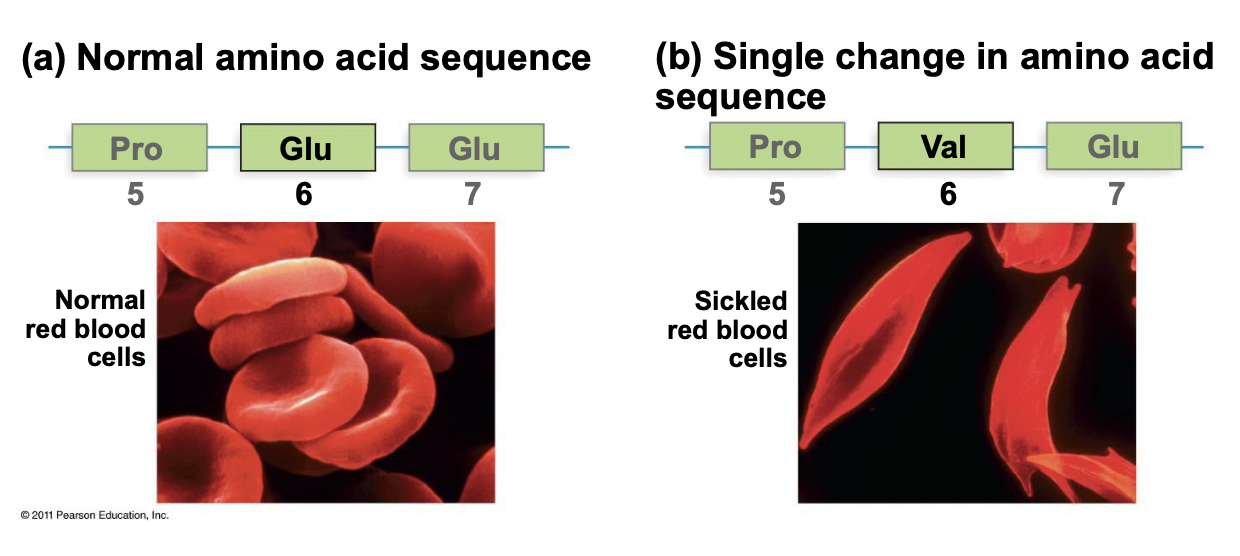
Amino Acid Sequence Importance
* Proteins diluted in watery solution denature at high temperature (unfold)
* Will renature (refold) when temperature is lowered
* Thus, the primary structure is sufficient for folding and the function is encoded in the amino acid sequence
* Ex. 1 amino acid mutation in hemoglobin causes sickle cell anemia
* Will renature (refold) when temperature is lowered
* Thus, the primary structure is sufficient for folding and the function is encoded in the amino acid sequence
* Ex. 1 amino acid mutation in hemoglobin causes sickle cell anemia
7
New cards
Protein Turnover
* Breakdown and resynthesis, occurs constantly in cells
* Every protein has a half life
* Half life: the time after which half the proteins have broken down
* Every protein has a half life
* Half life: the time after which half the proteins have broken down
8
New cards
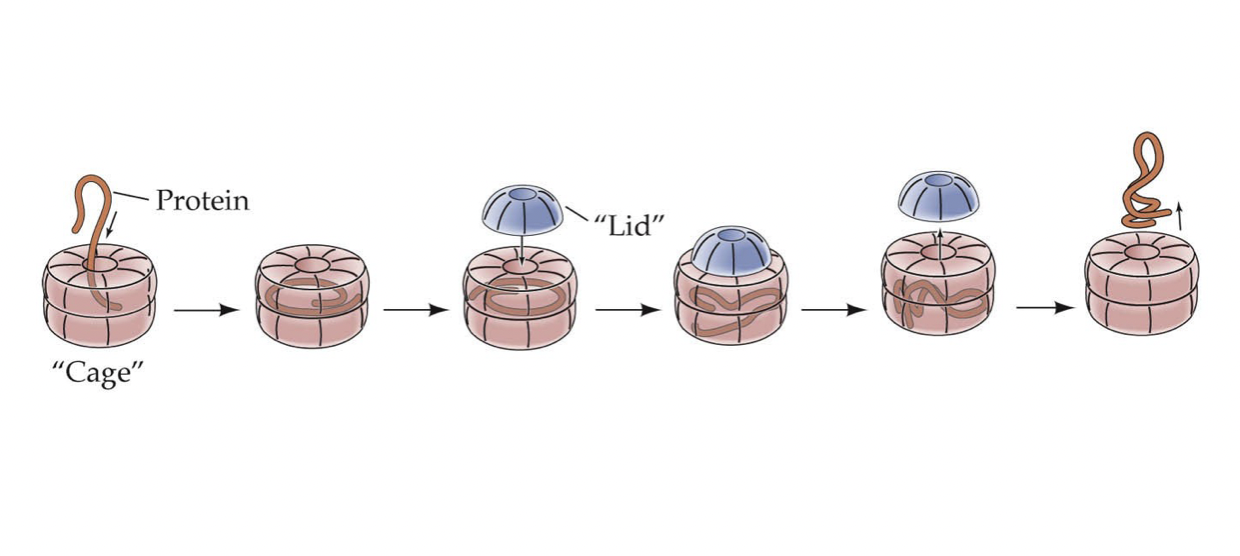
Chaperones
* Help proteins fold properly after synthesis or unfolding
* Keep other proteins from interacting inappropriately with eachother
* Keep other proteins from interacting inappropriately with eachother
9
New cards
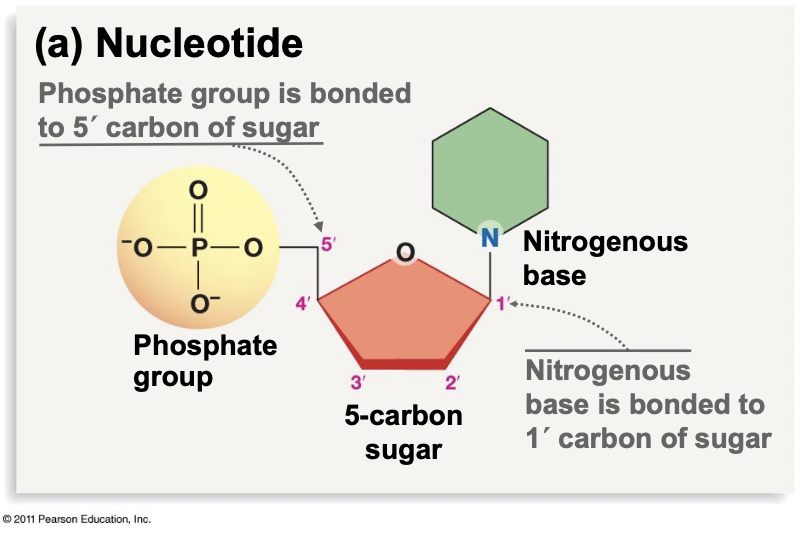
Nucleotides
* Occur in RNA, DNA, and energy carries (ex. ATP)
* Serve functions in signalling and energy storage as monomers
* Made of a phosphate group, attached to the 5’ carbon on a 5-carbon sugar, and the 1’ carbon of the sugar is bonded to a nitrogenous base
* Serve functions in signalling and energy storage as monomers
* Made of a phosphate group, attached to the 5’ carbon on a 5-carbon sugar, and the 1’ carbon of the sugar is bonded to a nitrogenous base
10
New cards
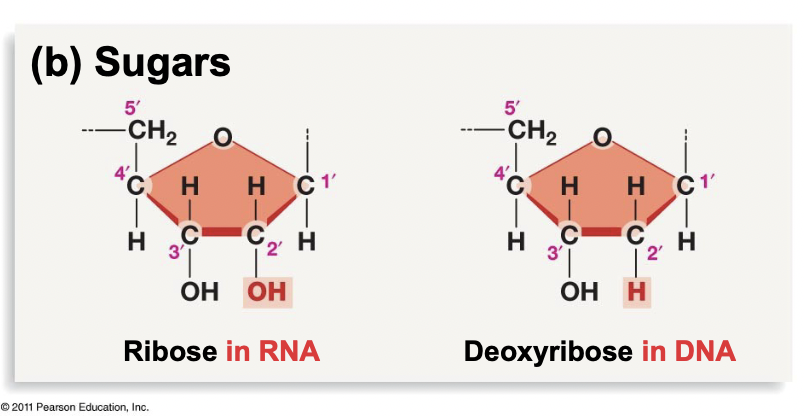
Carbon Sugar in RNA vs. DNA
* RNA: called ribose, with an OH attached to the 2’ carbon
* DNA: called deoxyribose, with an H attached to the 2’ carbon
* No oxygen makes it more stable
* DNA: called deoxyribose, with an H attached to the 2’ carbon
* No oxygen makes it more stable
11
New cards
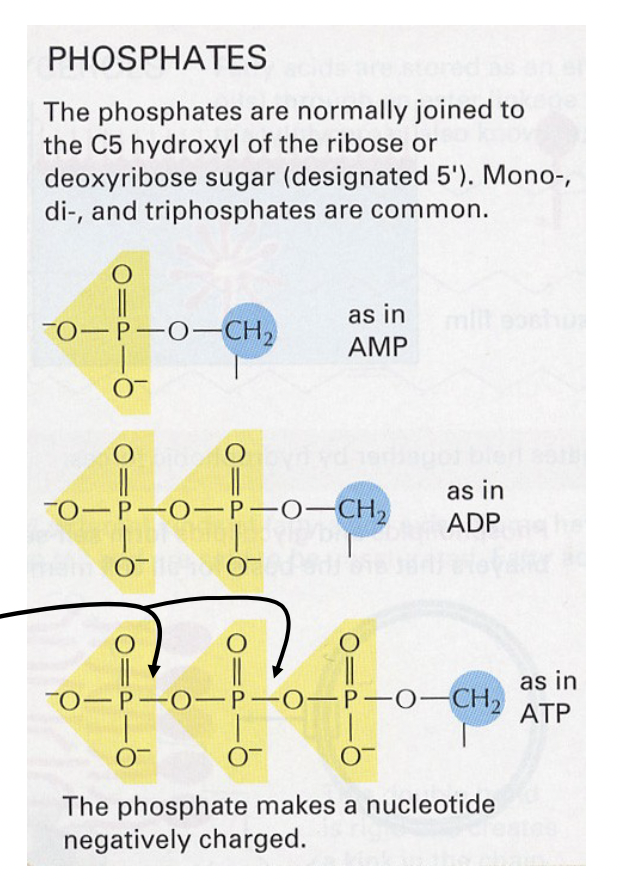
Phosphates in Nucleotides
* Can be monophosphate, diphosphate, or triphosphate, for a single nucleotide in solution
* Connects with the 5’ carbon on/in the sugar
* Considered energy rich bonds because their hydrolysis (depolymerization) releases energy
* ADP has 2, ATP has 3
* Connects with the 5’ carbon on/in the sugar
* Considered energy rich bonds because their hydrolysis (depolymerization) releases energy
* ADP has 2, ATP has 3
12
New cards
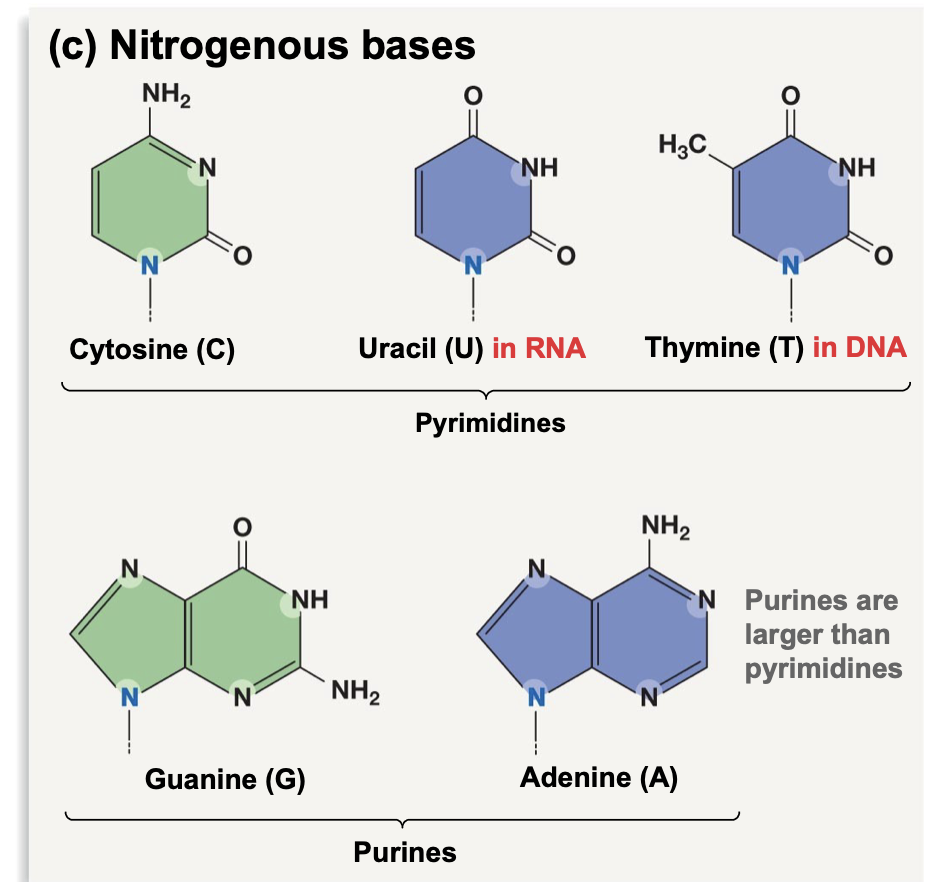
Nitrogenous Bases
Pyrimidines: single aromatic ring
* Cytosine, Uracil, Thymine
Purines: two aromatic rings (larger)
* Guanine, Adenine
\*\*In RNA Thymine is replaced by Uracil
* Cytosine, Uracil, Thymine
Purines: two aromatic rings (larger)
* Guanine, Adenine
\*\*In RNA Thymine is replaced by Uracil
13
New cards
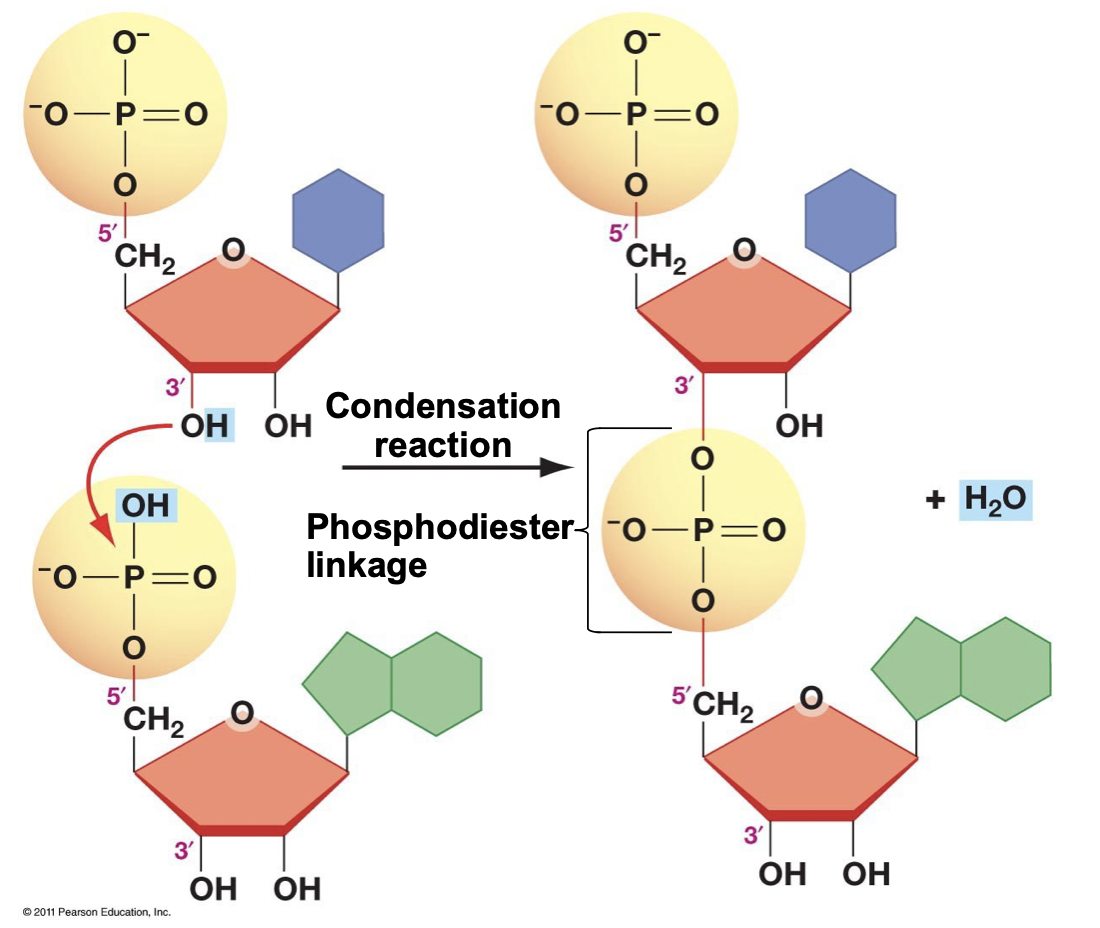
Phosphodiester Linkage
* When nucleotides polymerize (condensation reaction)
* Polymerization occurs 5’ to 3’ direction
* The 5’ phosphate on the incoming molecule forms a covalent bond with the 3’ hydroxyl
* Thus, RNA/DNA molecules start with a 5’ phosphate and end with a 3’ hydroxyl
* Polymerization occurs 5’ to 3’ direction
* The 5’ phosphate on the incoming molecule forms a covalent bond with the 3’ hydroxyl
* Thus, RNA/DNA molecules start with a 5’ phosphate and end with a 3’ hydroxyl
14
New cards
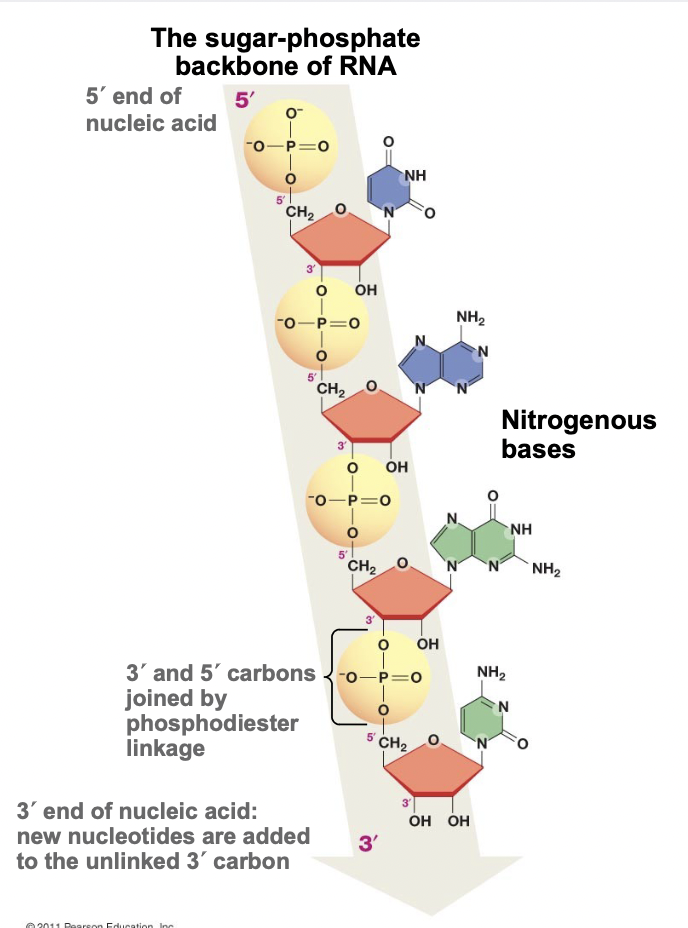
Sugar Phosphate Backbone
* The backbone of RNA and DNA molecules, long chain of nucleotides
* Made by phosphodiester linkage of nucleotides
* The nitrogenous bases do not play a role in formation
* Faces the outside while nitrogenous bases face inside
* Protects them from reacting with other molecules, makes D/RNA stable
* Made by phosphodiester linkage of nucleotides
* The nitrogenous bases do not play a role in formation
* Faces the outside while nitrogenous bases face inside
* Protects them from reacting with other molecules, makes D/RNA stable
15
New cards
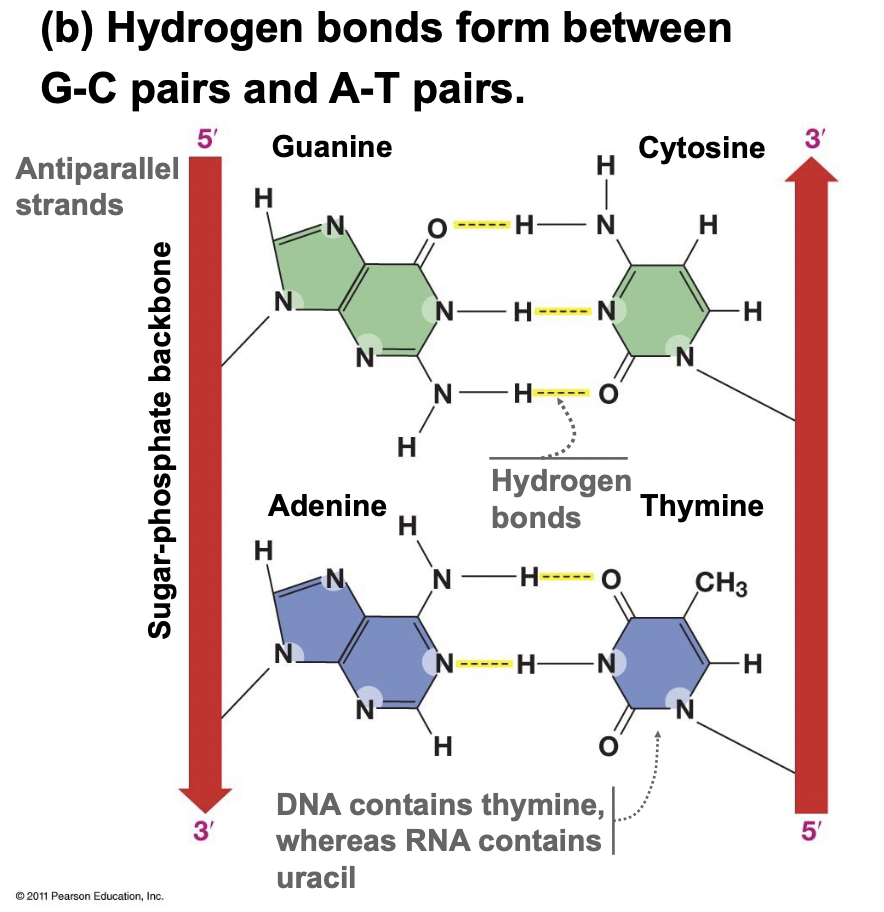
Nitrogenous Base Pairing Rules
* Purine bases only pair with pyrimidine bases
* Guanine → Cytosine form 3 H-bonds (most stable)
* Adenine → Thymine form 2 H-Bonds (RNA)
* Adenine → Uracil form 2 H-Bonds (RNA)
* Guanine → Cytosine form 3 H-bonds (most stable)
* Adenine → Thymine form 2 H-Bonds (RNA)
* Adenine → Uracil form 2 H-Bonds (RNA)
16
New cards
RNA vs. DNA Structure
* RNA is made of one sugar phosphate backbone, has U not T, usually has a loop and folds over on itself
* DNA is made of two antiparallel strands, forming a helix
* RNA can occasionally execute info like proteins
* DNA is made of two antiparallel strands, forming a helix
* RNA can occasionally execute info like proteins
17
New cards
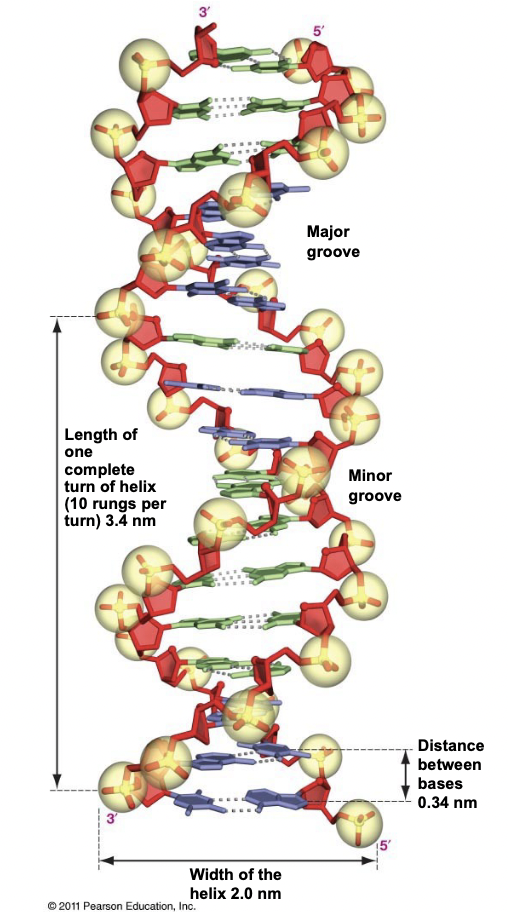
Major and Minor Grooves
* Occur in DNA double helices
* Major Groove: the 2 sugar-phos backbones are more widely spaced, allowing DNA-binding proteins to recognize the nitrogenous bases in the interior
* Important for transcription factors, enzymes etc.
* Major Groove: the 2 sugar-phos backbones are more widely spaced, allowing DNA-binding proteins to recognize the nitrogenous bases in the interior
* Important for transcription factors, enzymes etc.
18
New cards
Metabolism
All chemical reactions in a cell, divided into two types:
* Anabolic: make something, link simple molecules to make complex ones, energy storing reactions, require energy
* Catabolic: break down complex molecules into simpler ones, release energy
* Anabolic: make something, link simple molecules to make complex ones, energy storing reactions, require energy
* Catabolic: break down complex molecules into simpler ones, release energy
19
New cards
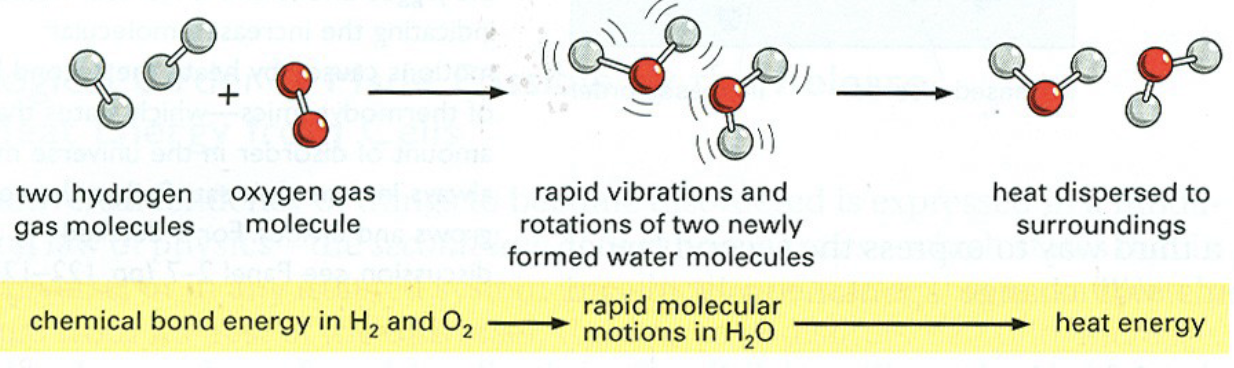
Energy Conversion in Chemical Reactions
chemical bond energy in molecules (potential) → rapid molecular motions while joining (kinetic) → heat energy (heat)
20
New cards
What drives energy conversions?
* Not energy content/potential energy
* The drive of energy to become evenly distributed/dispersed pushes reactions forward
* The drive of energy to become evenly distributed/dispersed pushes reactions forward
21
New cards
First Law of Thermodynamics
During any energy conversion, the total initial energy is the same as the total final energy, as energy cannot be created or destroyed.
22
New cards
Second Law of Thermodynamics
Energy spontaneously disperses from being localized to becoming spread out if it is not hindered from doing so, thus entropy increases.
Or, energy transformations always result in a state of higher probability (a more disordered state).
Or, energy transformations always result in a state of higher probability (a more disordered state).
23
New cards
What is change in entropy/overall disorder in the universe?
The amount of energy released to drive a chemical reaction.
24
New cards
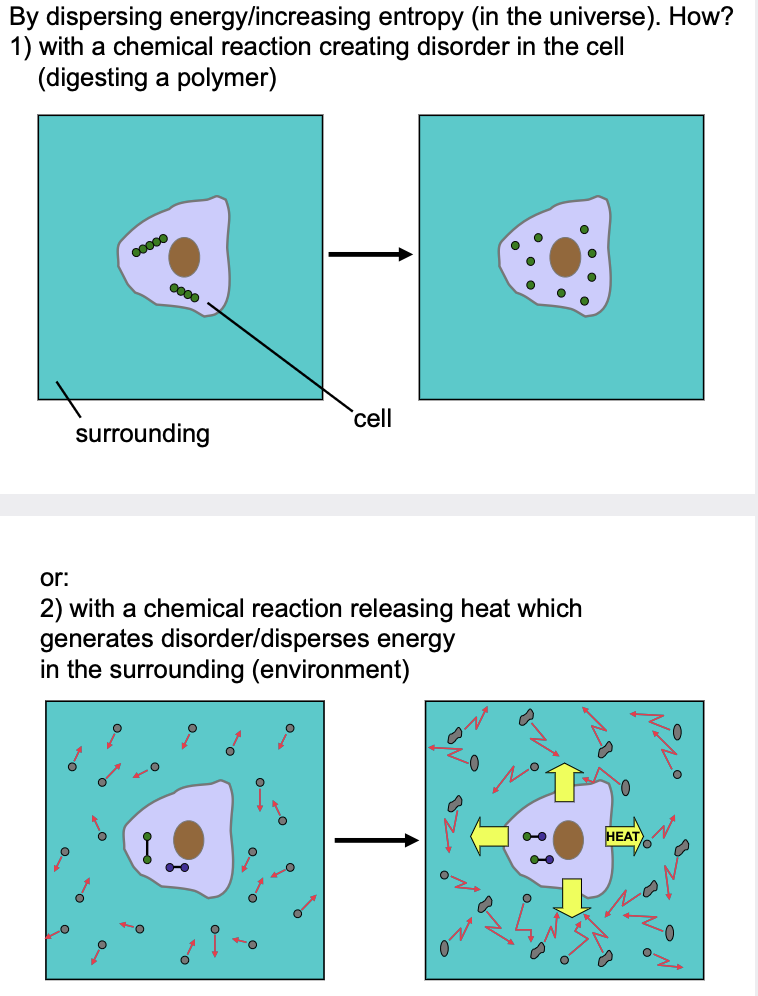
What are the two ways a cell can release free energy (drive a chemical reaction)?
1) Entropy (S) - directly increasing/creating disorder in the cell (or closed system)
* Ex. digesting a polymer, cutting a protein
2) Enthalpy (H) - undergoing a chemical reaction that releases heat into the surroundings making the disorder occur outside the cell (or closed system)
* Ex. digesting a polymer, cutting a protein
2) Enthalpy (H) - undergoing a chemical reaction that releases heat into the surroundings making the disorder occur outside the cell (or closed system)
25
New cards
Total Free Energy and delta G Explained
* delta G: total free energy (cal or J)
* delta H: enthalpy
* delta S: entropy/disorder
* T: absolute temperature
If delta G is:
* Negative: energy is released (disorder created) and the reaction is favourable/spontaneous
* Positive: energy is required, not spontaneous
* delta H: enthalpy
* delta S: entropy/disorder
* T: absolute temperature
If delta G is:
* Negative: energy is released (disorder created) and the reaction is favourable/spontaneous
* Positive: energy is required, not spontaneous
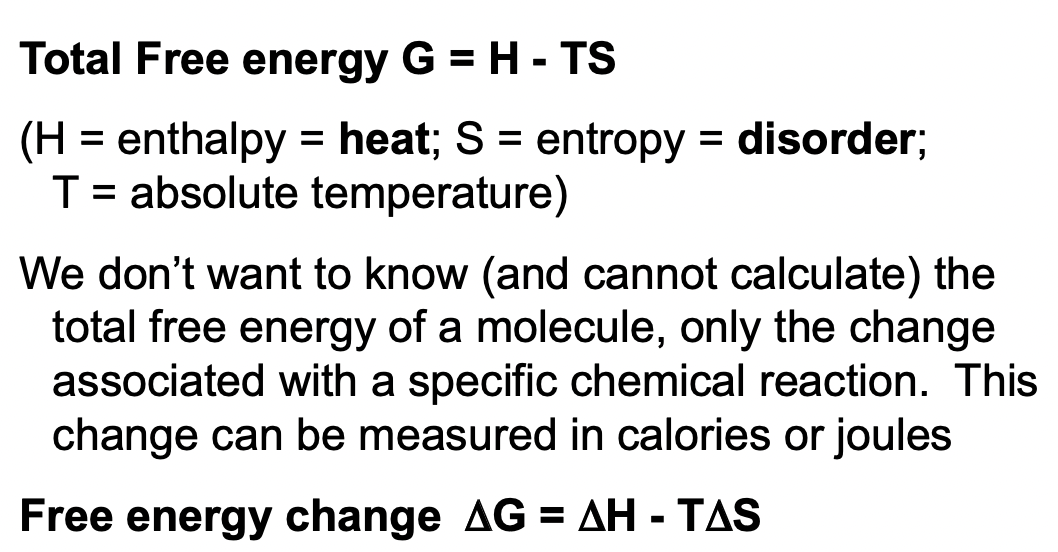
26
New cards
Four Types of Reactions
1. Exergonic: heat is released (-H), disorder created (+TS), spontaneous (-G)
* Most catabolic reactions
2. Heat is released (-H), disorder decreases (-TS), spontaneous (-G) ABOVE a certain temperature
* Ex. in protein folding heat is released, but disorder decreases because you get a nicely folded protein, if temperature is too high it will never fold and makes (+G)
3. Heat is used up (+H), disorder created (+TS), spontaneous (-G) ABOVE a certain temperature
* Ex. Dissolving NaCl in water since heat is needed to break the crystal lattice
4. Endergonic: heat is used up (+H), disorder decreases (-TS), never spontaneous (+G)
* Most anabolic reactions
27
New cards
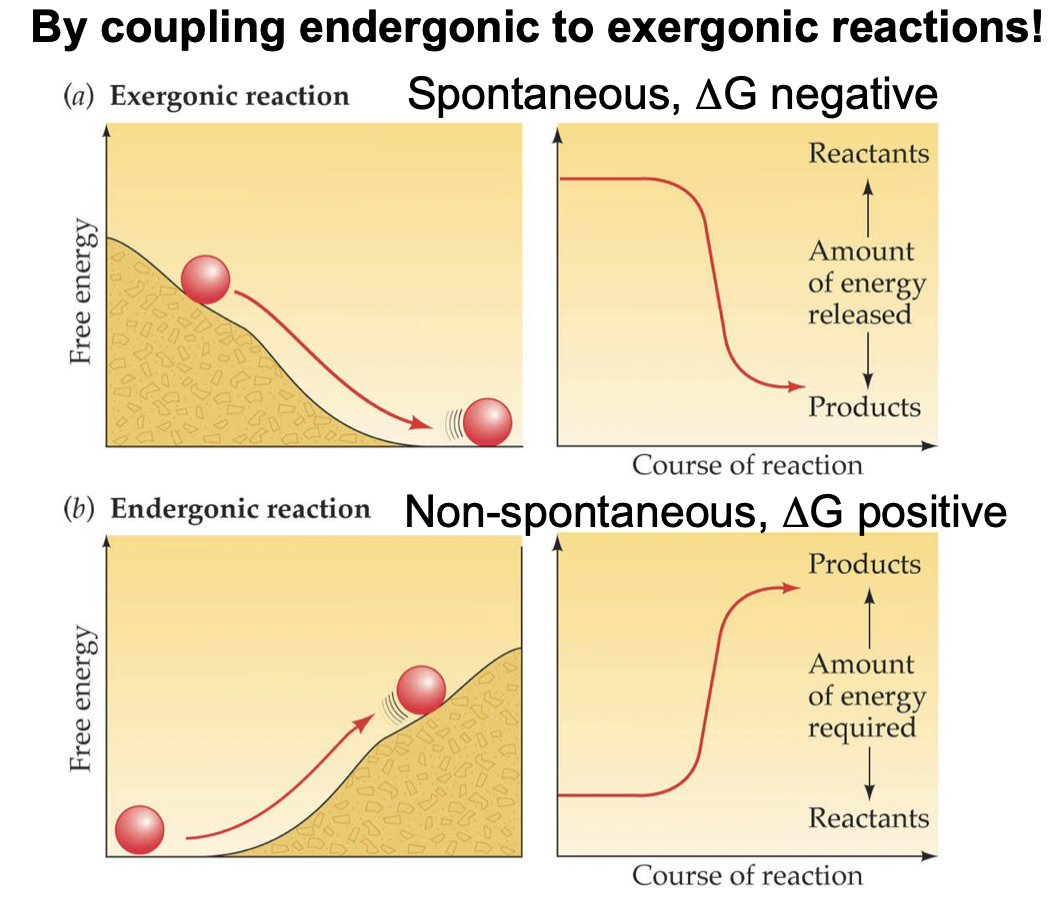
Reaction Coupling
Since anabolic (endergonic) reactions have a positive delta G, they must be coupled with catabolic (exergonic) reactions to make the overall delta G negative.
28
New cards
Equilibrium and Reversible Reactions
* All reactions are theoretically reversible, by the law of mass action
* If reactions are left alone, they reach an equilibrium point where forward and reverse reactions take place at the same rate (G=0)
* If reactions are left alone, they reach an equilibrium point where forward and reverse reactions take place at the same rate (G=0)
29
New cards
Energy Transfer in Cells
* All living cells use ATP for energy capture, transfer, and storage
* Some of the free energy released by exergonic reactions is captured in ATP, which then can drive endergonic reactions
* Some of the free energy released by exergonic reactions is captured in ATP, which then can drive endergonic reactions
30
New cards
ATP Hydrolysis
* ATP hydrolyzes to yield ADP and an inorganic phosphate ion
* This reaction is very exergonic since the body has a very high ATP concentration and the ADP concentration is very low
* This reaction is very exergonic since the body has a very high ATP concentration and the ADP concentration is very low
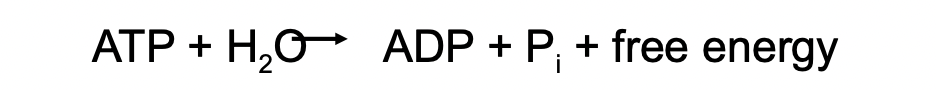
31
New cards
ATP and ADP Reaction Cycle
* ADP + Pi → ATP: Endergonic, energy required, so needs an exergonic reaction to provide energy
* ATP → ADP + Pi: Exergonic, energy released, so needs an endergonic reaction to give the energy to
* ATP → ADP + Pi: Exergonic, energy released, so needs an endergonic reaction to give the energy to
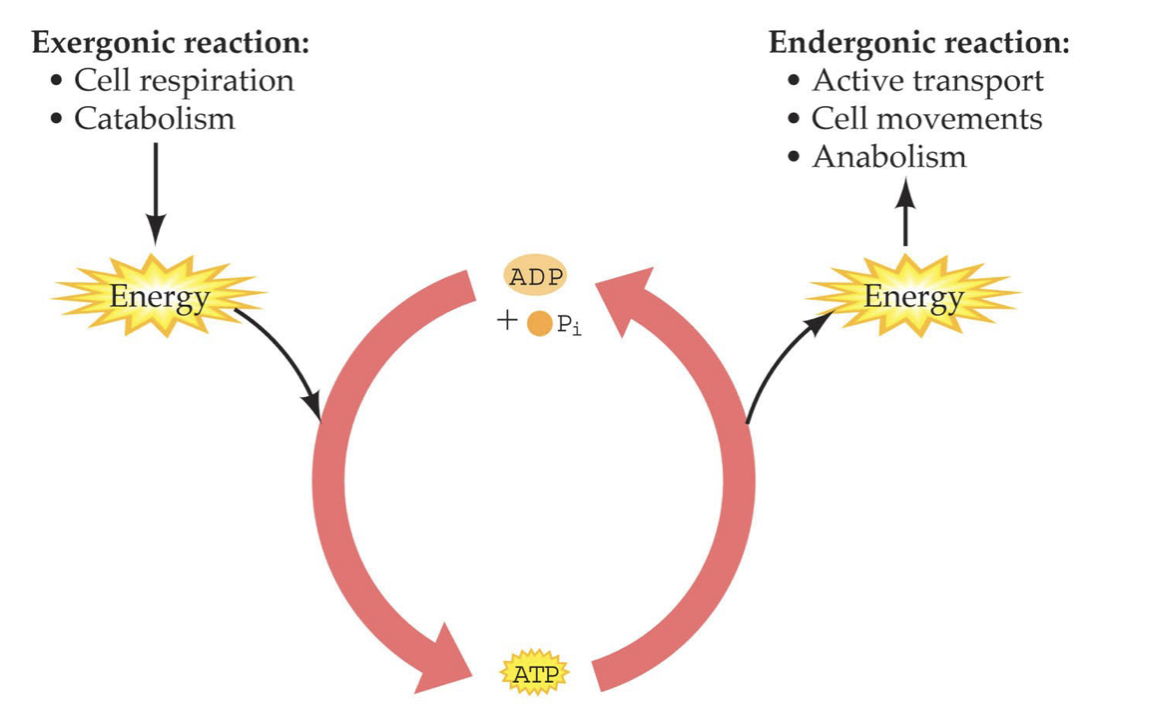
32
New cards
What can you predict about a reaction?
* Direction of a reaction can be predicted if the delta G is known, but not the rate of the reaction
33
New cards
Activation Energy and Relevance to Exergonic Reactions
Activation Energy: the amount of energy needed to put molecules into a transition state (less stable state)
* Exergonic reactions proceed only after the activation energy is added
* Exergonic reactions proceed only after the activation energy is added
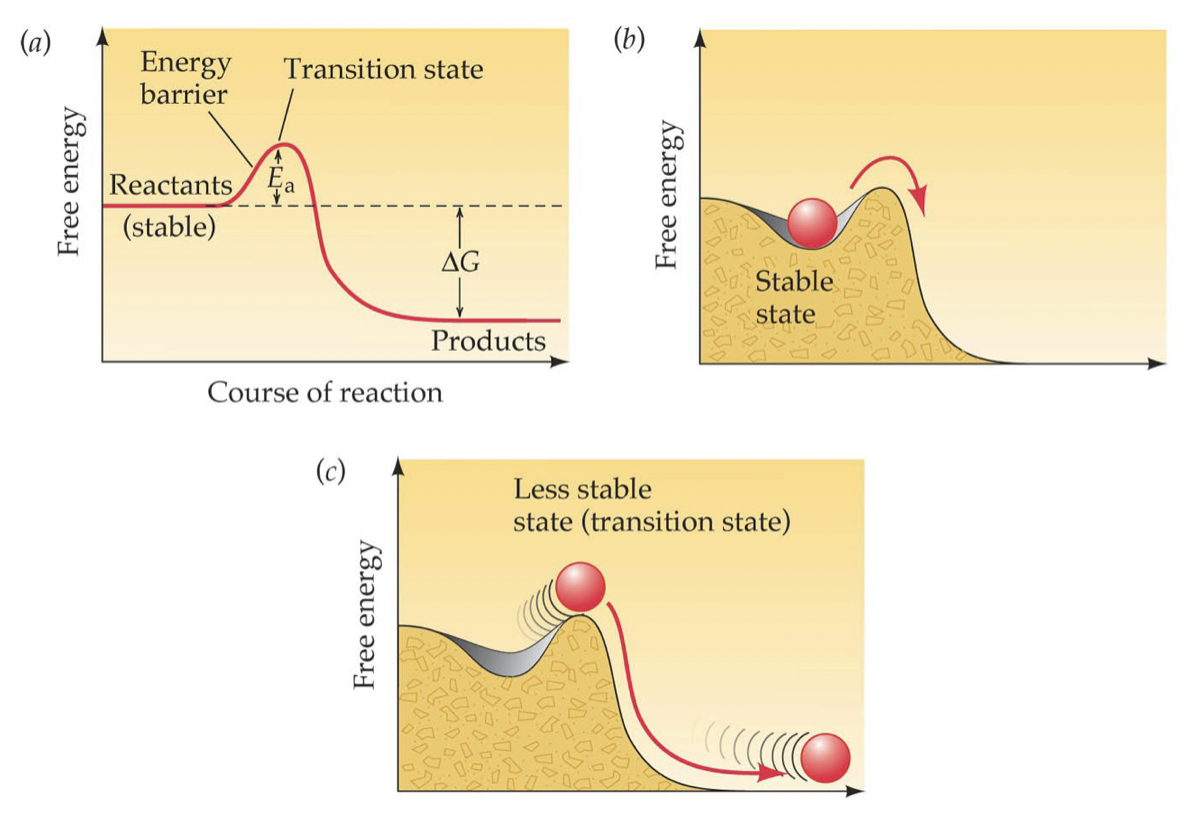
34
New cards
Catalysts
* Substance that seeds up a reaction but is not used up in it (ex. enzymes)
* Only applicable to reactions with overall negative delta G
* Delta G is not changed, only lowers the activation energy required
* Only applicable to reactions with overall negative delta G
* Delta G is not changed, only lowers the activation energy required
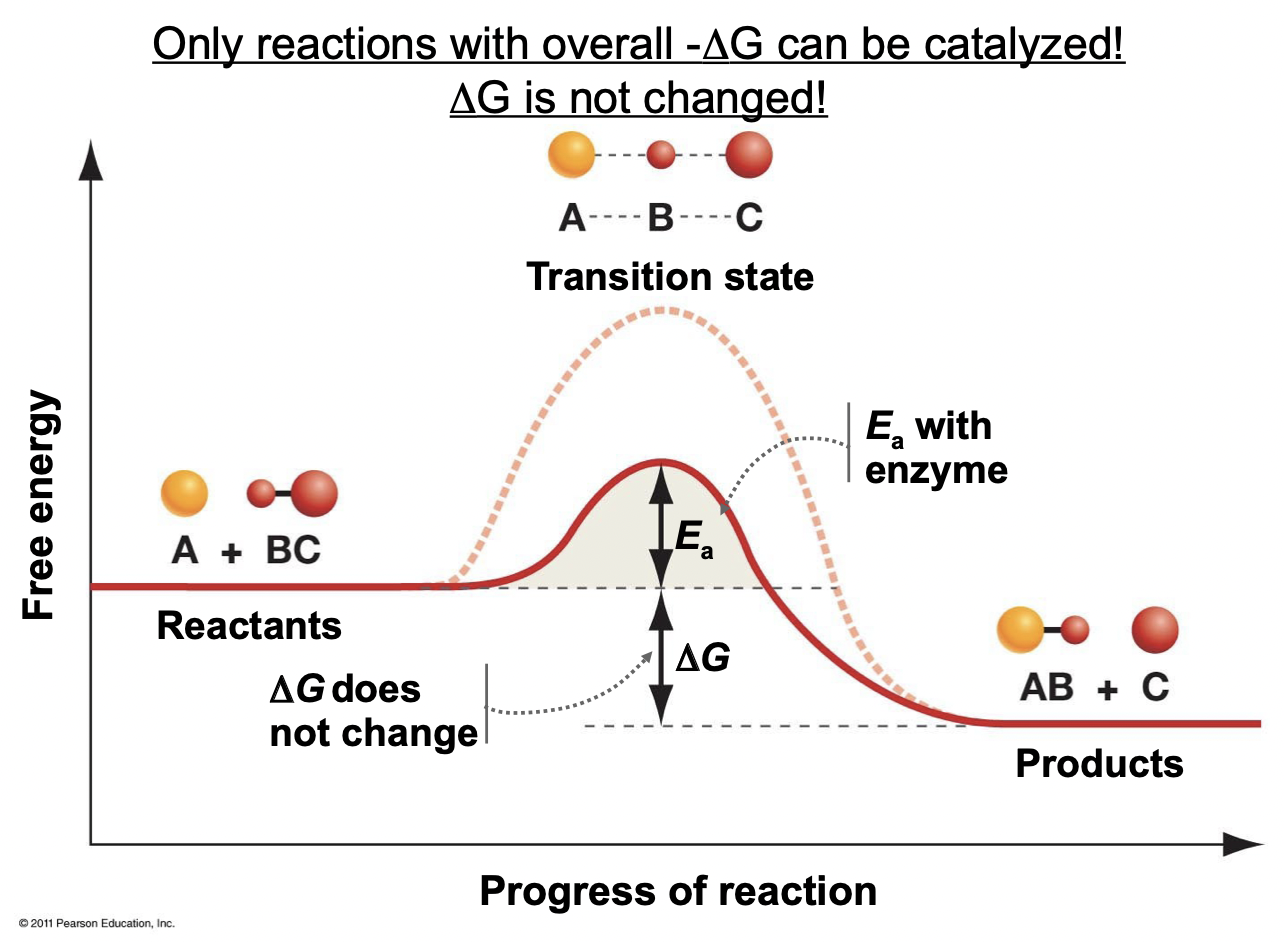
35
New cards
Explain Enzyme-Substrate Interactions
* Reactants (substrates) bind to the active enzyme site
* Enzyme undergoes conformational change that brings the substrates into a transition state, with lower activation energy, speeding up the reaction
* Products leave the active site, and the enzyme reverts to its original shape, unchanged
* Enzyme undergoes conformational change that brings the substrates into a transition state, with lower activation energy, speeding up the reaction
* Products leave the active site, and the enzyme reverts to its original shape, unchanged
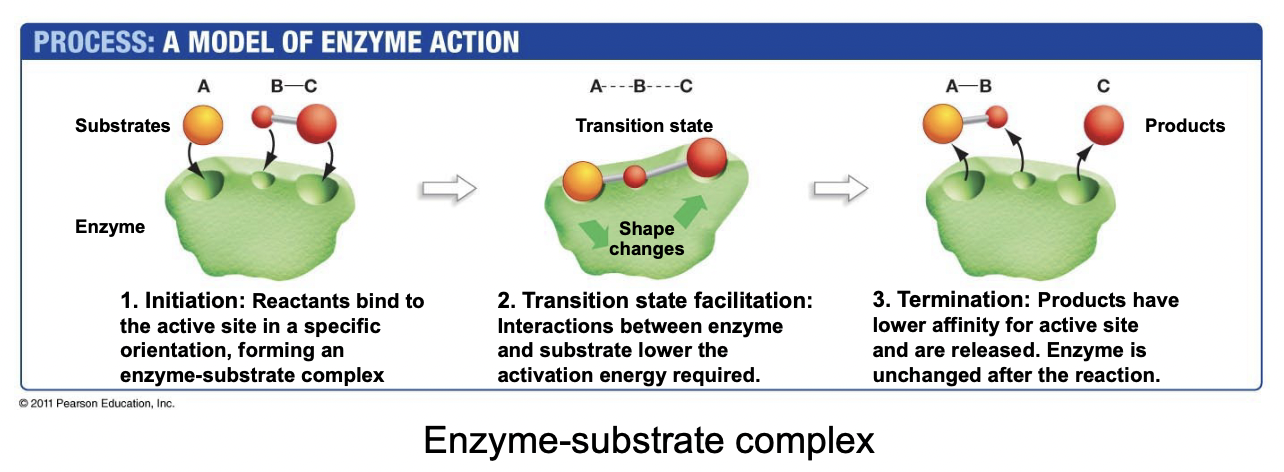
36
New cards
How can you induce the transition state of a substrate?
* Binding them in the correct orientation
* Exposing reactants to a differently charged environment, promoting catalysis
* Inducing a strain on the substrate to break a covalent bond
* Exposing reactants to a differently charged environment, promoting catalysis
* Inducing a strain on the substrate to break a covalent bond
37
New cards
Enzyme Cofactors
* Some enzymes require cofactors to function
* Can be metal ions (zinc), small organic molecules (not amino acids), temporarily or permanently bonded
* Can be metal ions (zinc), small organic molecules (not amino acids), temporarily or permanently bonded
38
New cards
What does it mean when an enzyme is saturated?
* All active sites are occupied
* A higher substrate concentration does not increase the rate of product formation
* The maximum reaction rate is achieved
* A higher substrate concentration does not increase the rate of product formation
* The maximum reaction rate is achieved
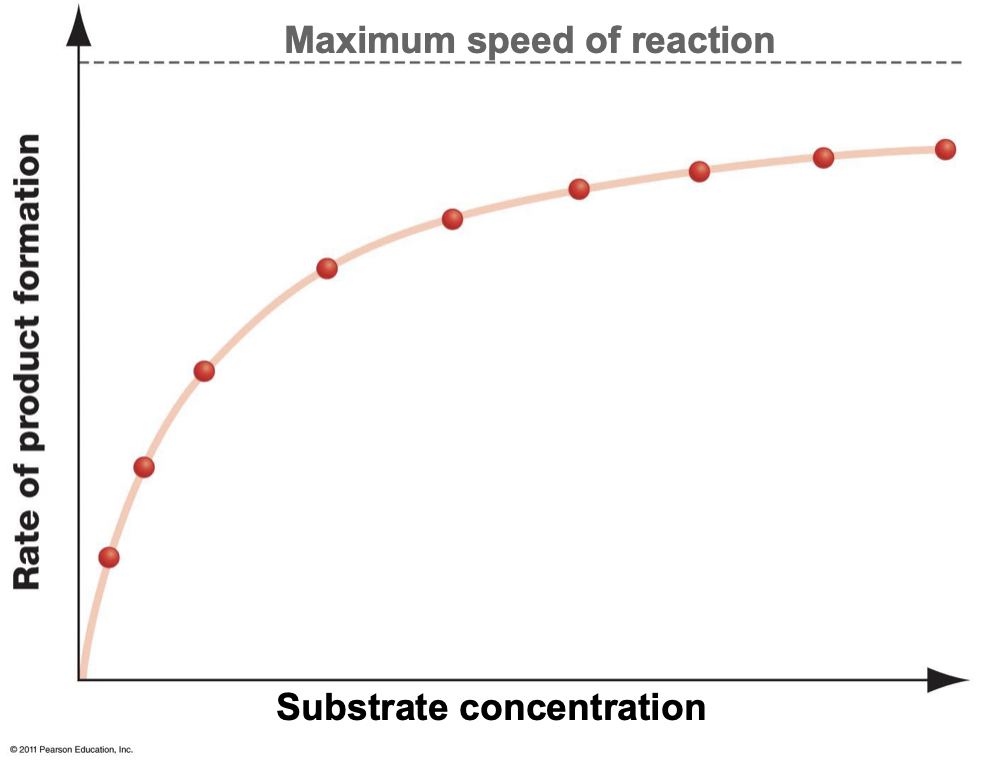
39
New cards
Three Categories of Carbohydrates
* Monosaccharides
* Disaccharides: two monosaccharides
* Polysaccharides: 3 or more monosaccharides (up to 100,000s)
* Disaccharides: two monosaccharides
* Polysaccharides: 3 or more monosaccharides (up to 100,000s)
40
New cards
Functions of Sugar
Energy storage, building block for nucleic acids, structural component (ex. in wood)
41
New cards
General Sugar Formula & Structure
* Multiples of CH2O
* An aldose, carbonyl group at the end of the carbon chain
* A ketose, carbonyl group in the middle of the carbon chain
* Aldose and ketose are isomers
* An aldose, carbonyl group at the end of the carbon chain
* A ketose, carbonyl group in the middle of the carbon chain
* Aldose and ketose are isomers
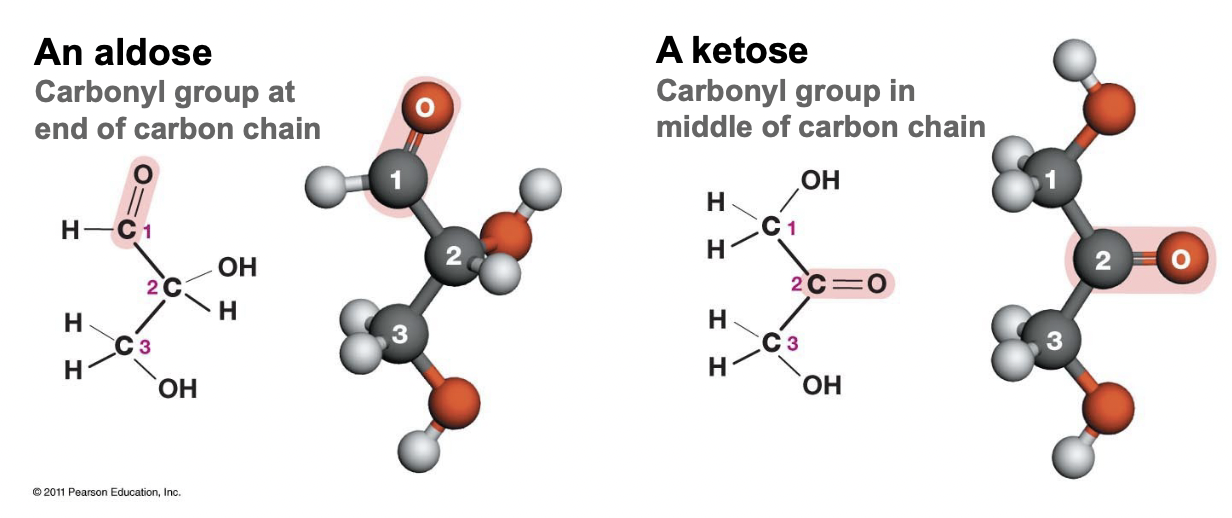
42
New cards
Isomers
* Compounds that have the same molecular formula but are structurally different
* Usually a functional group attached to a different carbon
* Usually a functional group attached to a different carbon
43
New cards
Optical Isomers
* Occur when a carbon has four different groups attached (chiral centre)
* Non-super imposable mirror images of each other
* Non-super imposable mirror images of each other
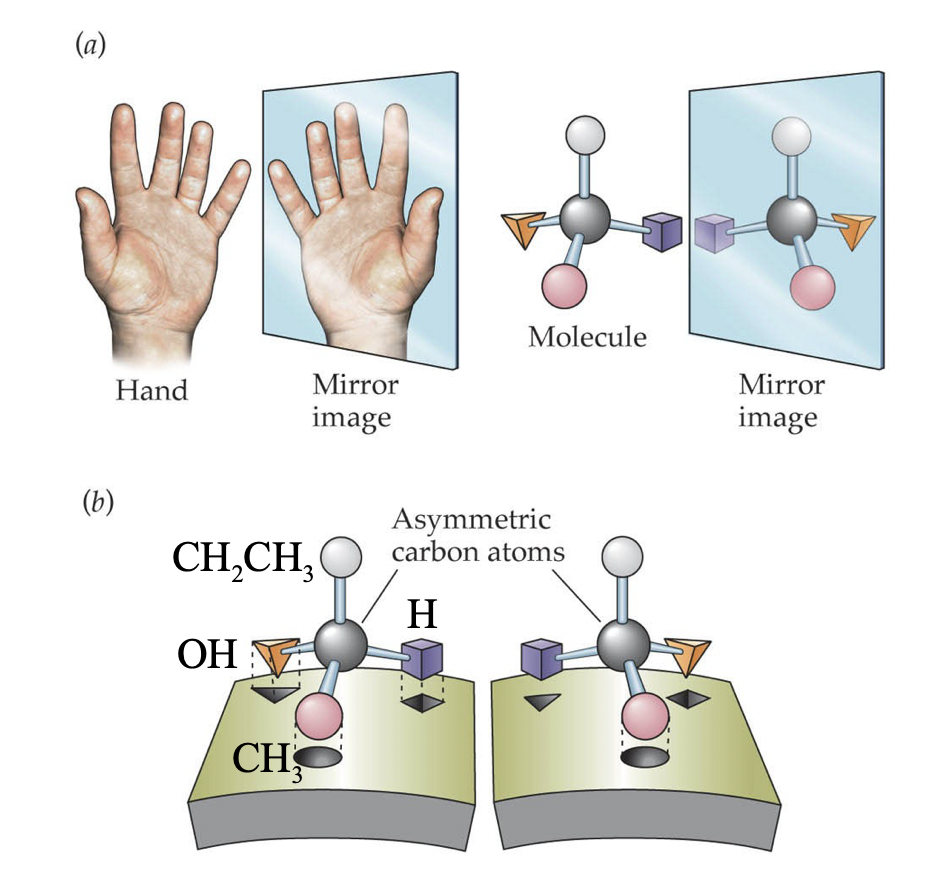
44
New cards
Glucose and Galactose
* Optical Isomers of each other
* The hydroxyl on the fourth carbon is flipped
* The hydroxyl on the fourth carbon is flipped
45
New cards
What happens to glucose in solution?
* The straight chain forms another covalent bond, becoming a ring
* This makes the first carbon asymmetric, giving two isomers
* Alpha and beta glucose
* When the molecule is a monomer it can change between both isomers freely, it links and unlinks
* This makes the first carbon asymmetric, giving two isomers
* Alpha and beta glucose
* When the molecule is a monomer it can change between both isomers freely, it links and unlinks
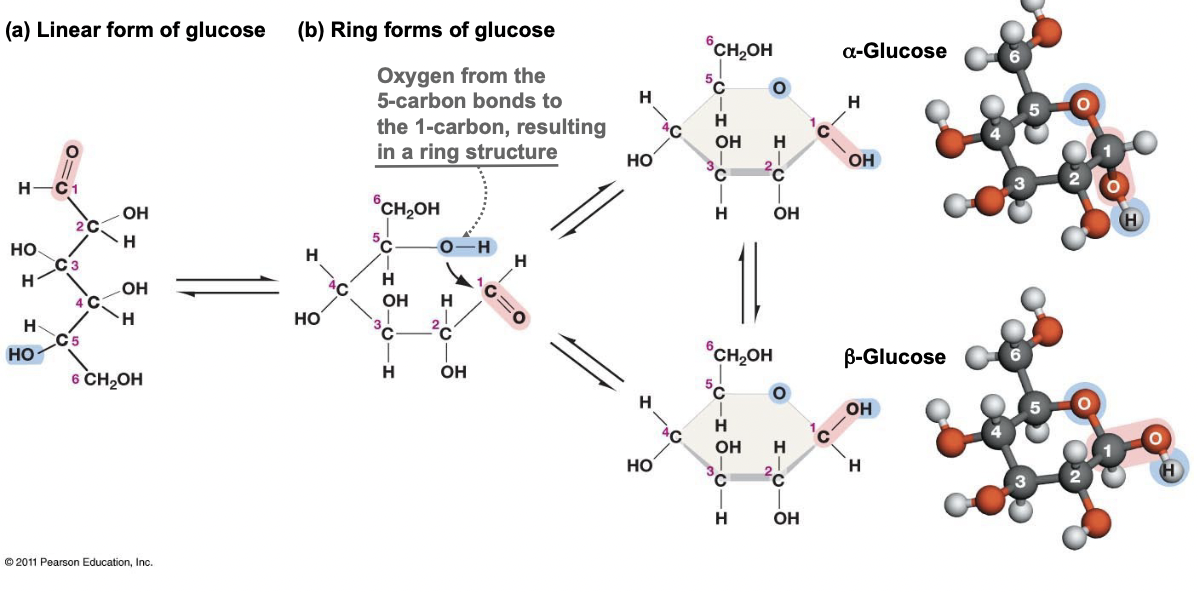
46
New cards
Glycosidic Linkage
* When 2 glucose monomers link through the oxygen on the fourth carbon on the incoming molecule
* The oxygen attaches to the first carbon of the other molecule, replacing the OH
* The OH and the H on the incoming molecule form one molecule of water
* Once monomers link in a beta or alpha orientation they can no longer change
* The oxygen attaches to the first carbon of the other molecule, replacing the OH
* The OH and the H on the incoming molecule form one molecule of water
* Once monomers link in a beta or alpha orientation they can no longer change
47
New cards
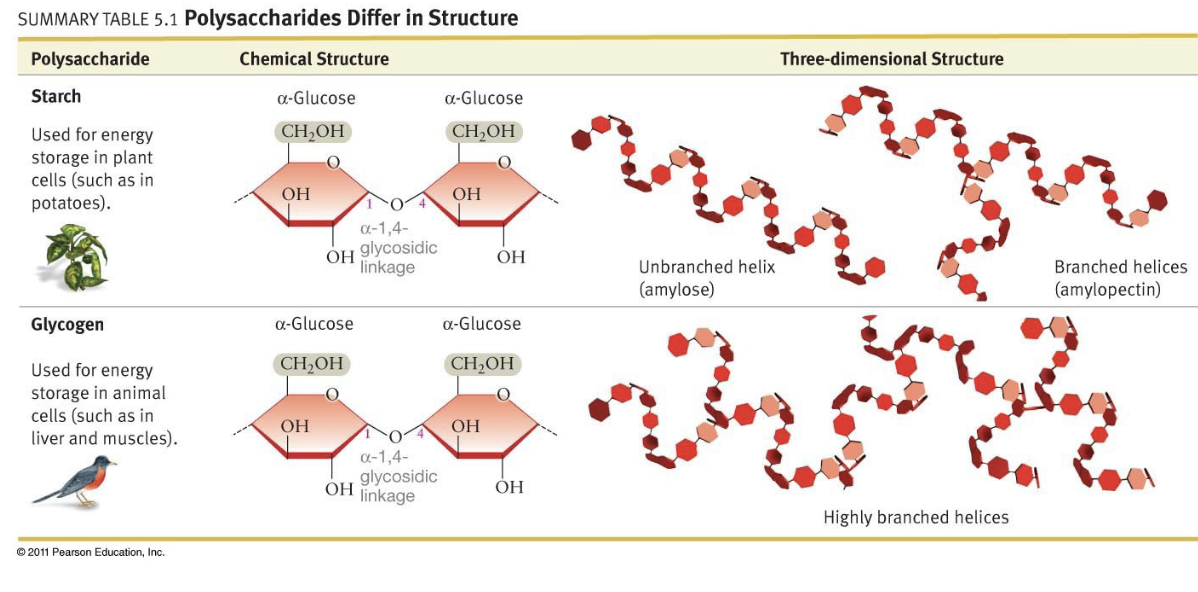
Glucose Alpha 1-4 Linkage, Properties in Plants vs. Animals
* 2 glucose molecules with alpha 1-4 linkage makes maltose
* More of these links make starch, links have same orientation
* Unbranched, in plants = amylose
* Moderately branched, in plants = amylopectin
* Lightly branched, in animals = glycogen
* Bulky CH2OH groups are all on the same side, resulting in the shape of a spiral
* More of these links make starch, links have same orientation
* Unbranched, in plants = amylose
* Moderately branched, in plants = amylopectin
* Lightly branched, in animals = glycogen
* Bulky CH2OH groups are all on the same side, resulting in the shape of a spiral
48
New cards
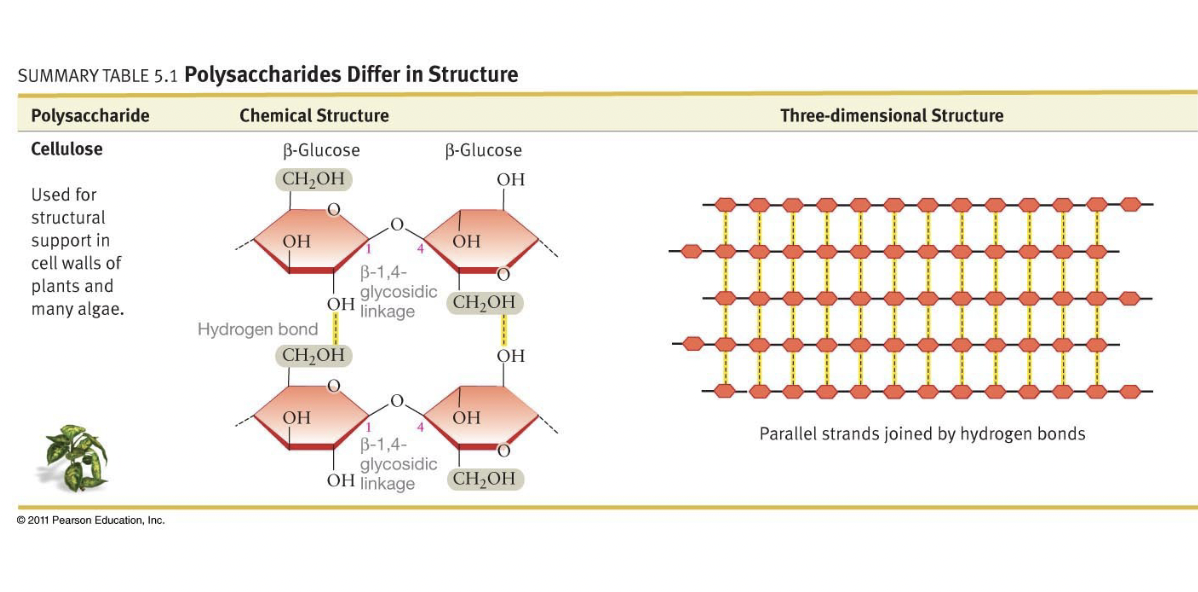
Glucose Beta 1-4 Linkage, Properties in Plants vs. Animals
* 2 glucose molecules with beta 1-4 linkage makes cellobiose
* More of these links makes cellulose, each link flips orientation
* Thus, linear, symmetrical, and always unbranched
* Better H-bonding and can form parallel strands
* More of these links makes cellulose, each link flips orientation
* Thus, linear, symmetrical, and always unbranched
* Better H-bonding and can form parallel strands
49
New cards
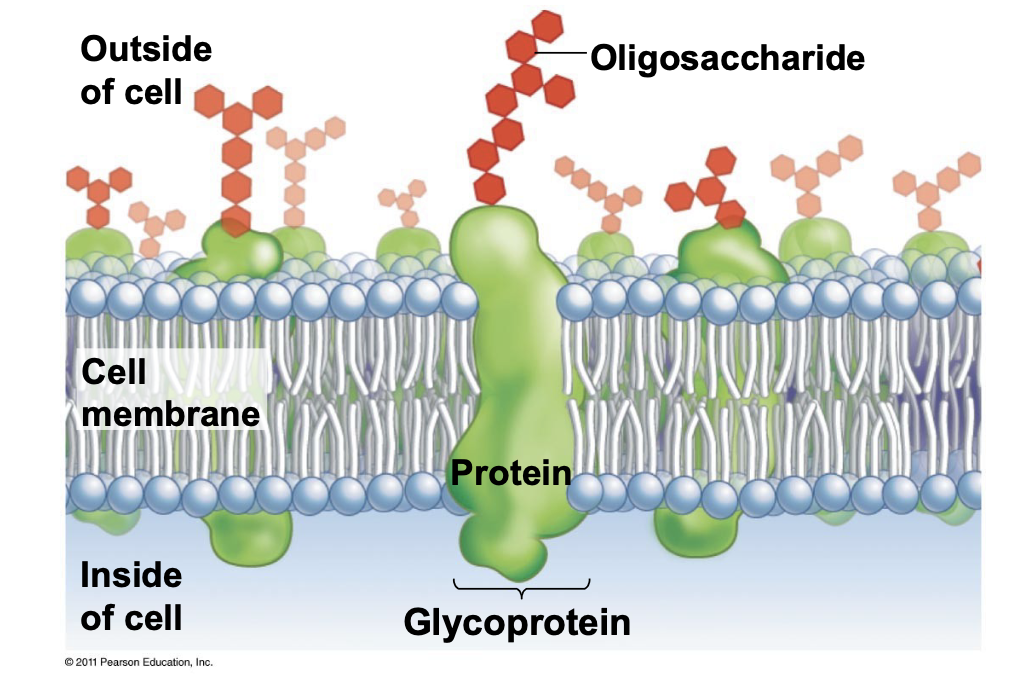
Oligosaccharide
10-20 sugar molecules linked together
50
New cards
Lipids
* Fats (solid) or oils (liquid)
* Hydrophobic, since few H atoms
* Most energy dense cell in the body
* Takes longer to mobilize than starch
* Hydrophobic, since few H atoms
* Most energy dense cell in the body
* Takes longer to mobilize than starch
51
New cards
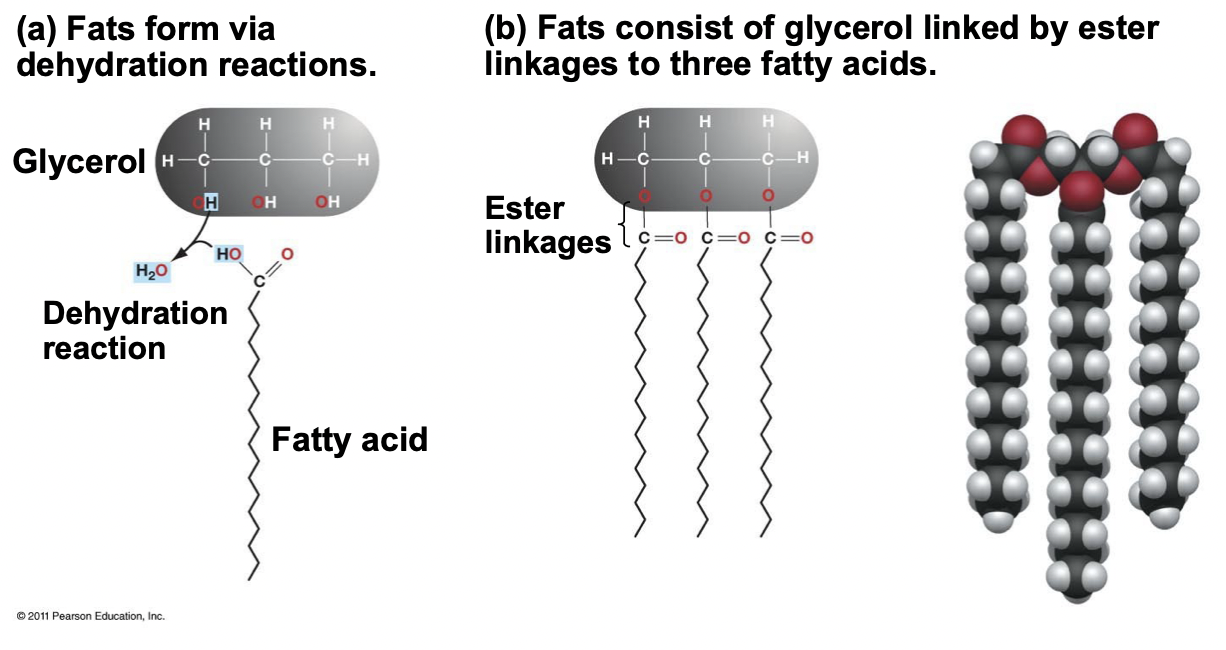
Fats and Oils Structure
Made of three fatty acids and one glycerol connected by ester linkages, formed through dehydration reactions.
* Fats have NO double bonds, packed together closer, van Der Waals is stronger
* Fats have AT LEAST one double bond, packed together less closely, van Der Waals is weaker
* Fats have NO double bonds, packed together closer, van Der Waals is stronger
* Fats have AT LEAST one double bond, packed together less closely, van Der Waals is weaker
52
New cards
Fatty Acids
* 1 carboxyl group and a long hydrocarbon chain
* Amphiphilic: hydrophobic hydrocarbon chain, hydrophilic carboxyl group
* Found in soap
* Amphiphilic: hydrophobic hydrocarbon chain, hydrophilic carboxyl group
* Found in soap
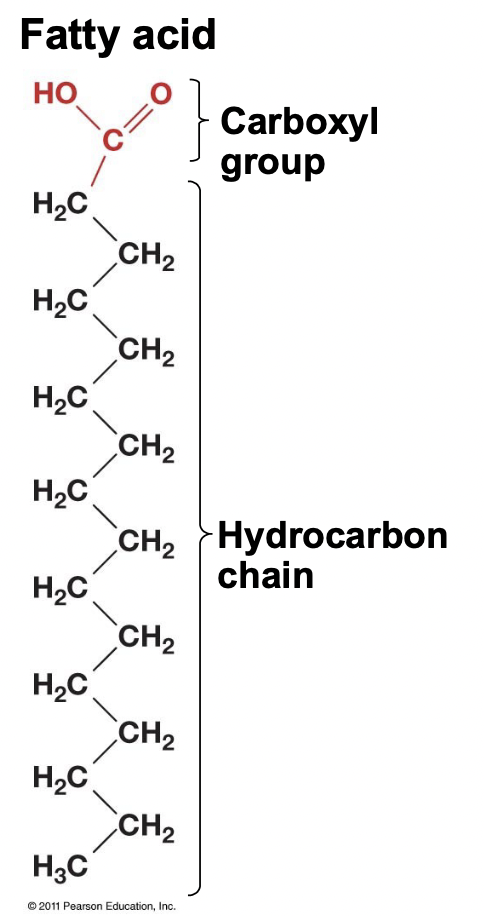
53
New cards
Phospholipids
* Very amphiphilic: hydrophilic head group, attached to glycerol, attached to 2 fatty acid tails
* Have the ability to self-assemble into lipid bilayers
* Have the ability to self-assemble into lipid bilayers
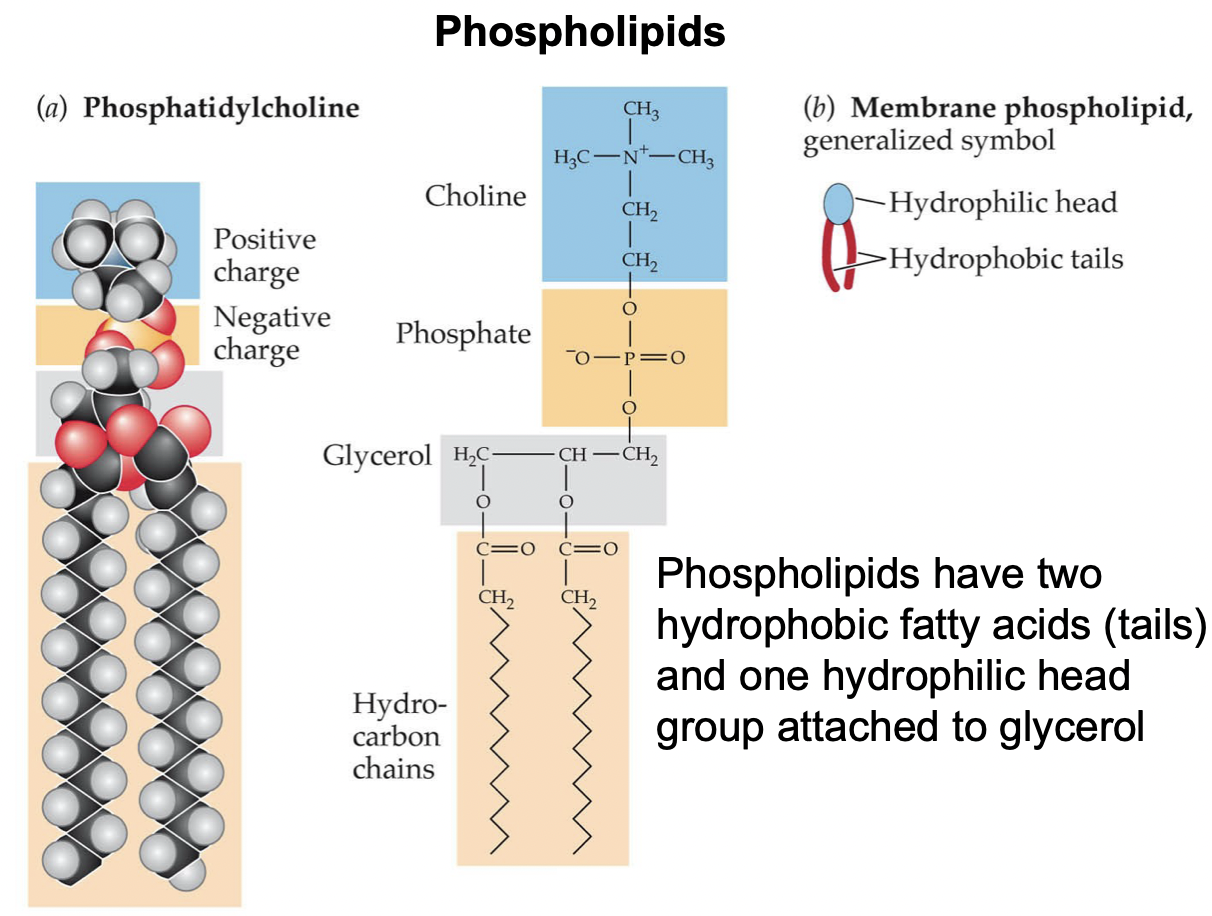
54
New cards
Lipid Bilayers
* Phospholipid heads interact with water (hydrophilic)
* Tails face inwards (hydrophobic)
* Movement in lateral direction, rarely flip to other side
* Bilayer sheets form a sealed compartment/sphere that is energetically favourable
* Tails face inwards (hydrophobic)
* Movement in lateral direction, rarely flip to other side
* Bilayer sheets form a sealed compartment/sphere that is energetically favourable
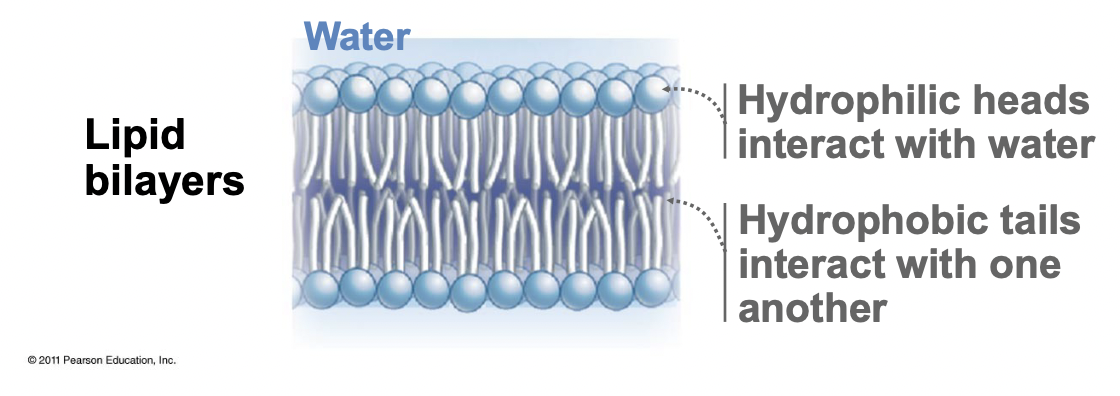
55
New cards
Unsaturated Fatty Acid
* Contain a double bond, creating a kink in the tail
* Cannot be packed as close together
* Increasing fluidity and permeability of the membrane
* Cannot be packed as close together
* Increasing fluidity and permeability of the membrane
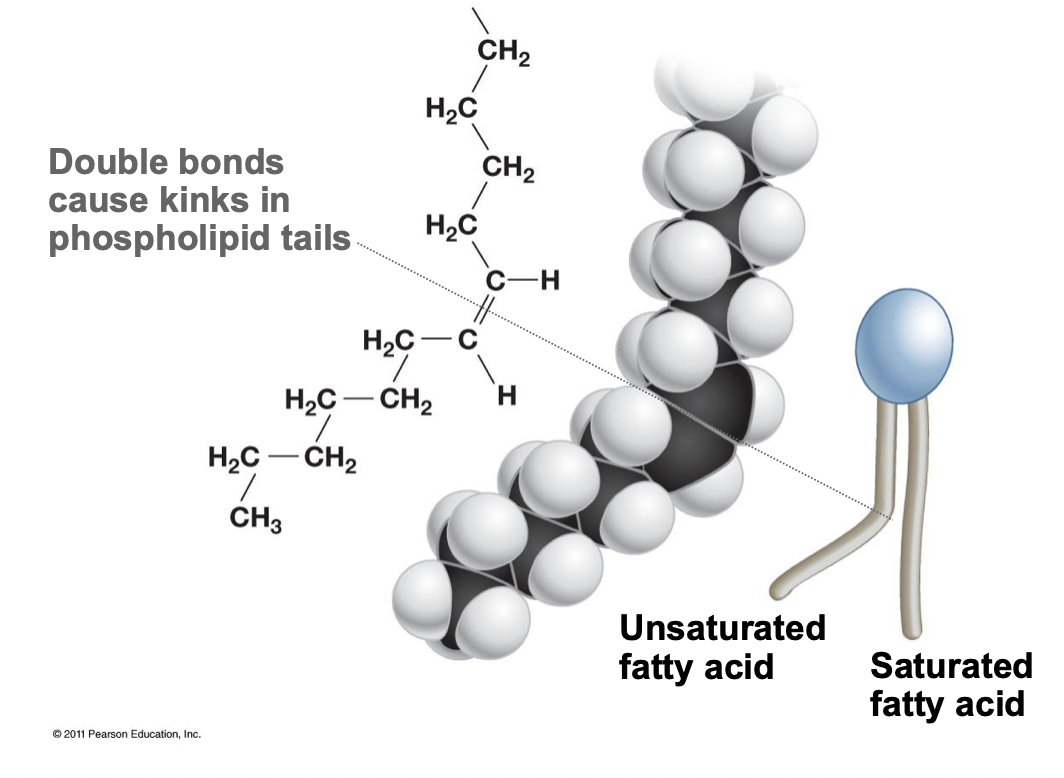
56
New cards
Unsaturated Fatty Acids in Fish and Plants
Fish and plants adjust the number of double bonds in phospholipids to keep membrane fluidity stable over a variety of temperatures.