Redox Chemistry & Electrochemical Cells
1/80
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
81 Terms
What is a redox reaction?
A redox reaction is a reaction where electron transfer between reactants is taking place. Some elements are said to be oxidized while others are being reduced
OIL RIG
What is the oxidizing agent?
An oxidising agent or oxidant is a substance that causes the oxidation of another, which means it accepts electron so the other substance can lose electrons
An oxidant itself being reduced.
What is the reducing agent?
An reducing agent or reductant is a substance that causes the reduction of another, which means it donates electrons so the other substance can gain electrons
An reductant is being oxidised
What is combustion?
A redox reaction involving fuel, which is either hydrogen gas (H2), methane (CH4), petrol, ethane (C2H6), ethanol (C2H6OH) is being oxidised and oxygen gas is being reduced
What is the combustion redox reaction equation (ethanol and ethane)?
C2H5OH(aq) + 3O2(g) —> 2CO2(g) + 3H2O(l)
Carbon is being oxidized and oxygen is being reduced.
2C2H6(aq) + 7O2(g) —> 4CO2(g) + 6H2O(l)
What is a corrison reaction?
A corrosion reaction is a type of redox reaction where a metal (iron, aluminum or zinc) is oxidized, typically by oxygen (this is being reduced) in the presence of moisture (water helps transfer electrons between the metal and oxygen, accelerating rusting), leading to the deterioration (rusting) of the metal. This process often results in the formation of metal oxides or other compounds.
What is the corrosion redox reaction equation?
2Fe(s) + 2H2O(l) + O2(g) —> 2Fe(OH)2(s)
What is metal displacement reaction?
A metal displacement reaction is a type of redox reaction where a more reactive metal loses a electron to a less reactive metal from its compound in solution, resulting in the formation of a new metal and a new compound (transfer from one metal element to the metal ions of a less reactive metal).
More reactive metal is oxidized (positive metal ions)
Less reactive metal is reduced (gains electrons)
How does a metal reactivity increases (how can you tell on the periodic table)?
Down a group (e.g., Group 1 and 2): Reactivity increases as atoms get larger and lose electrons more easily.
Across a period (left to right): Reactivity decreases because atoms hold onto electrons more tightly
What is the metal displacement redox reaction (copper and iron)?
Cu(s) + 2Ag+(aq) —> 2Ag(s) + Cu2+(aq)
2Fe(s) + 3SnCl2(aq) —> 2FeCl3(aq) + 3Sn(s)
What is halogen displacement reaction?
Electron are transferred to a more reactive halogen (group 17) from a hallide ion of a less reactive halogen
More reactive halogen (higher up group 17) becomes reduced (gains electron and forms a negative halide ion)
Less reactive halogen becomes oxidized (lose electrons)
What is the halogen displacement redox reaction (for chlorine and bromine)?
Br2(l) + 2KI(aq) —> 2KBr(aq) + I2(l)
2Br-(aq) + Cl2(aq) —> 2Cl-(aq) + Br2(aq)
What is not a redox reaction?
Acid and base reaction (neutralisation reaction)3
What is an electrochemical cell?
An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it.
What are the two types of electrochemical cells?
Galvanic cell is an electrochemical cell which is able to generate an electrical current from a spontaneous chemical reaction (through the movement of electrons between electrodes), which occurs naturally without any external energy (galvanic cells are also known as batteries).
Electrolytic cell is an electrochemical cell which uses an electrical current to make a non-spontaneous reaction (a reaction that does not occur naturally and requires external energy input to proceed) go.
How does a galvanic cell work?
Galvanic cells use a redox reaction to produce voltage without direct contact between the oxidant and the reductant.
What is the anode and cathode in an galvanic cell?
An anode: where oxidation occurs and electrons are being released. It is in contact with the reducing agent.
•In galvanic cells, this is the negative electrode (repels electrons)
-The mass of the anode decreases after the substance is oxidized (solid to aqueous solution)
A cathode: where reduction occurs and electrons are being accepted. It is in contact with the oxidizing agent
•In galvanic cells, this is the positive electrode (attracts electrons)
-The mass of the cathode increases after the substance is reduced (aqueous solution becomes a solid)
Where do galvanic cell travel?
The electrons in a galvanic cell travel from the anode to the cathode.
A substance at the anode loses electrons to be gained by a substance at the cathode.
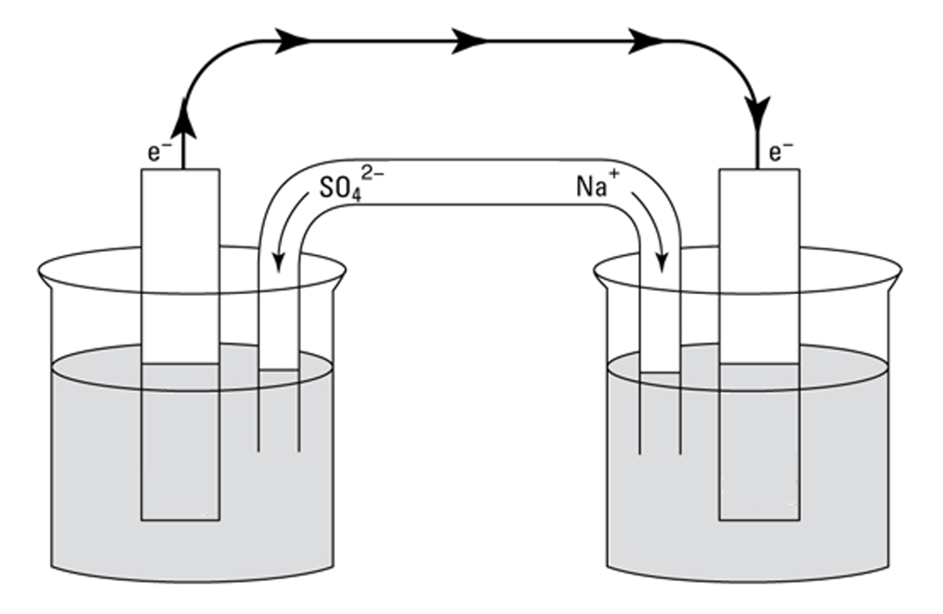
What is the salt bridge?
Connects the two solutions in a galvanic cell and allows ions to flow into both sides of the cell (two half-cells), which keeps both solutions neutral. Keeping the solution electrically neutral is necessary for electrons to travel from the anode to the cathode. (It is important to note that free electrons DO NOT travel through the salt bridge.)
What is the difference between an electrolyte and electrode?
An electrode is a conductor (often a metal) that provides a surface for electron transfer during oxidation or reduction, while an electrolyte is a substance (often a liquid) that provides the medium for ion transport
What is Daniell cell?
Uses copper sulfate as the oxidizing agent and zinc metal as the reducing agent.
Zinc metal (Zn) loses electrons and turns into zinc ions (Zn2+), which dissolve into the zinc sulfate solution. The electrons released by the oxidation of zinc and flow to the copper electrode
Copper ions from the electrolyte (ions dissolved in the liquid) move from to the copper electrode (conductor) to gain these electrons.
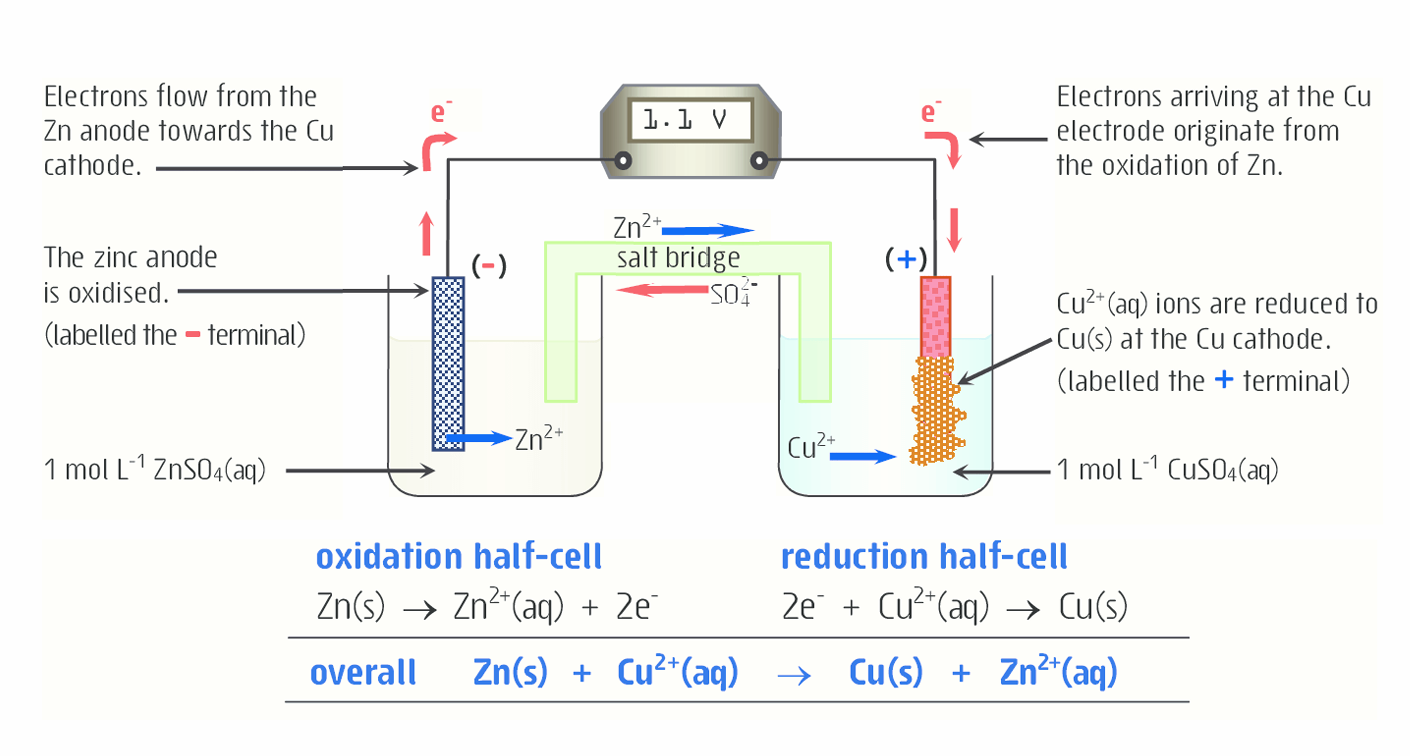
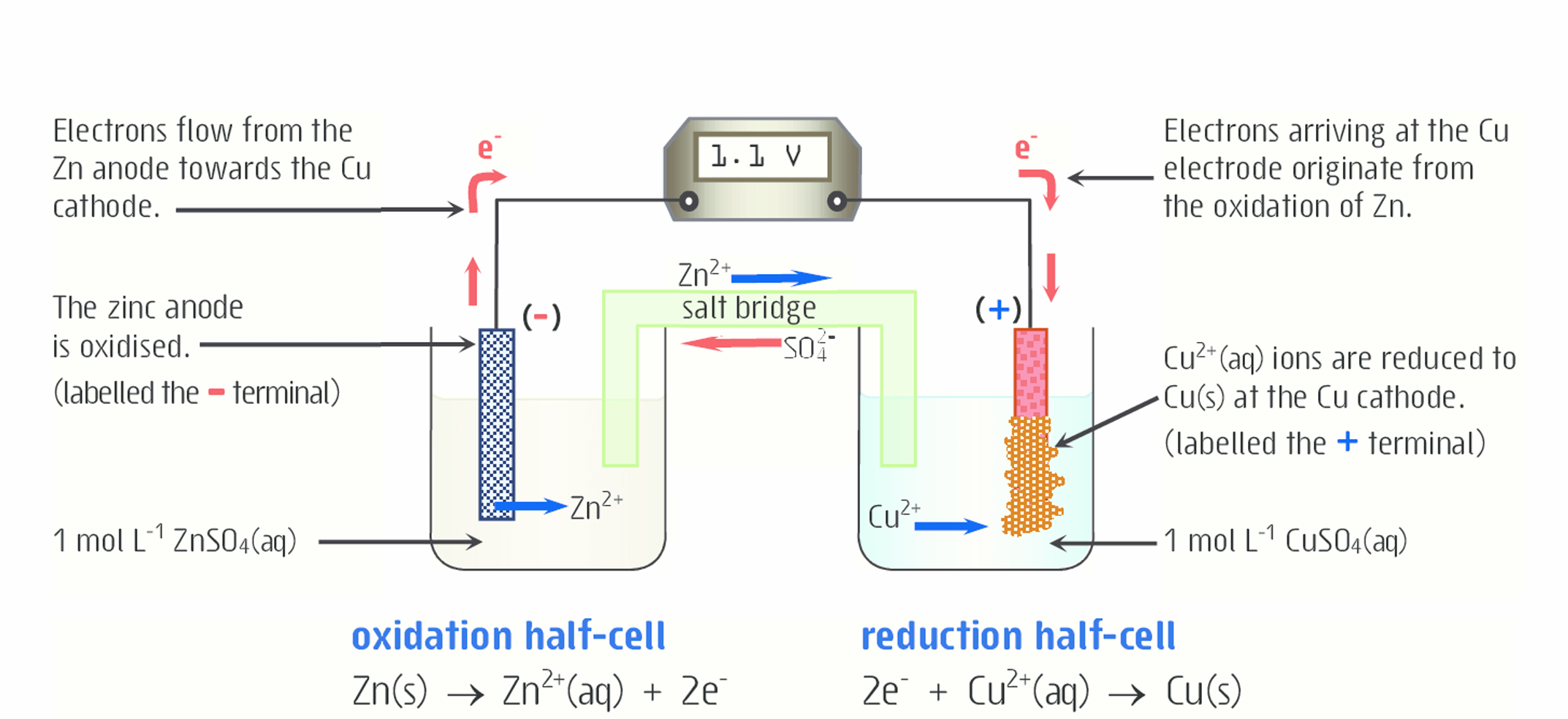
Explain what is happening to zinc in this diagram
Solid zinc is losing electrons and being oxidized to form Zn2+ , which moves from the electrode into the solution. In this case, the electrode is the anode. Oxidation always occurs at the anode.
The electrons from the oxidation of Zn2+ travel to aqueous Cu2+
The mass of the anode decreases, because as solid Zn is oxidized (loses electrons) to form Zn2+, it moves from the anode to the solution
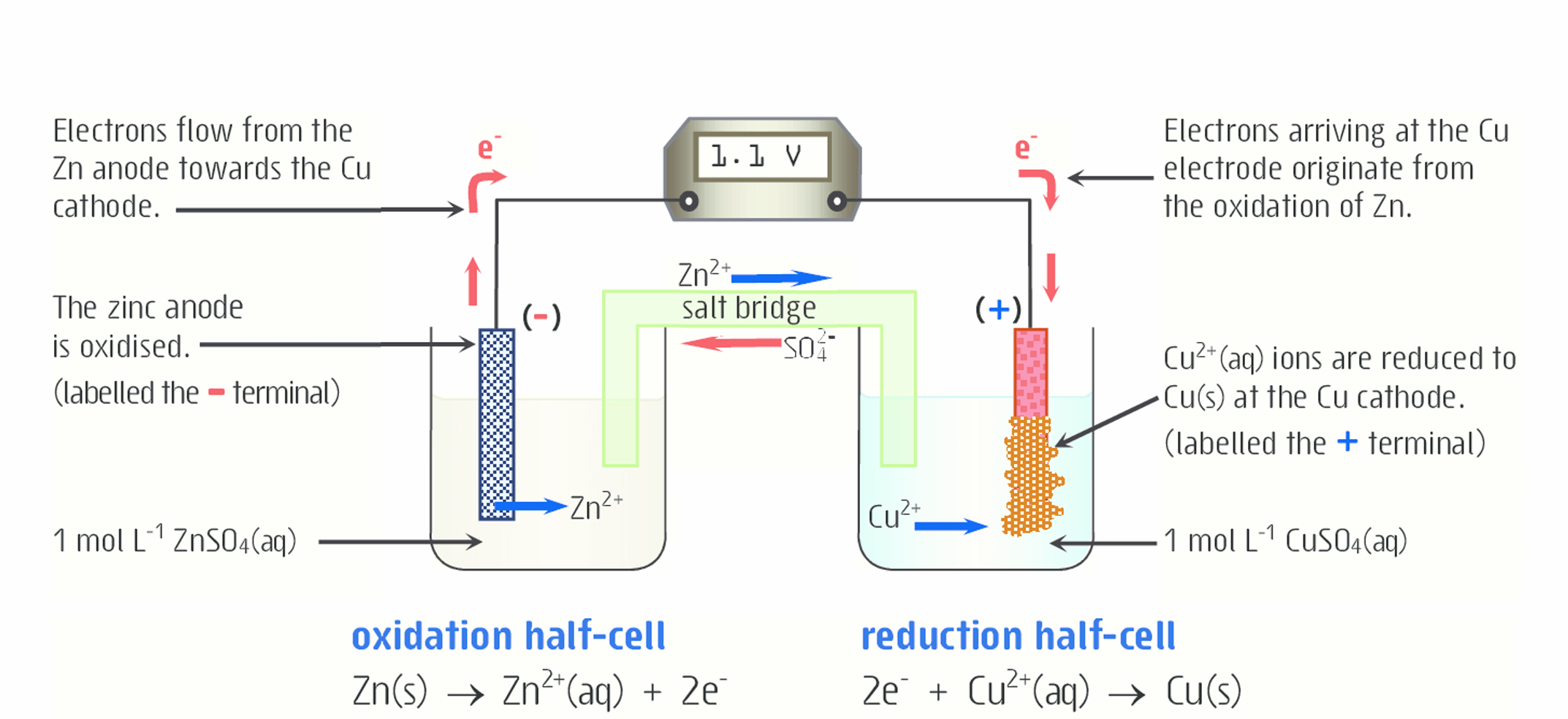
Explain what is happening to copper in this diagram
The aqueous Cu2+ is reduced (gains electrons) to form solid copper. It then moves to the electrode, in this case the cathode. Reduction always occurs at the cathode.
Because solid Cu is being added to the cathode as Cu2+ is reduced (gains electrons), the mass of the cathode increases.
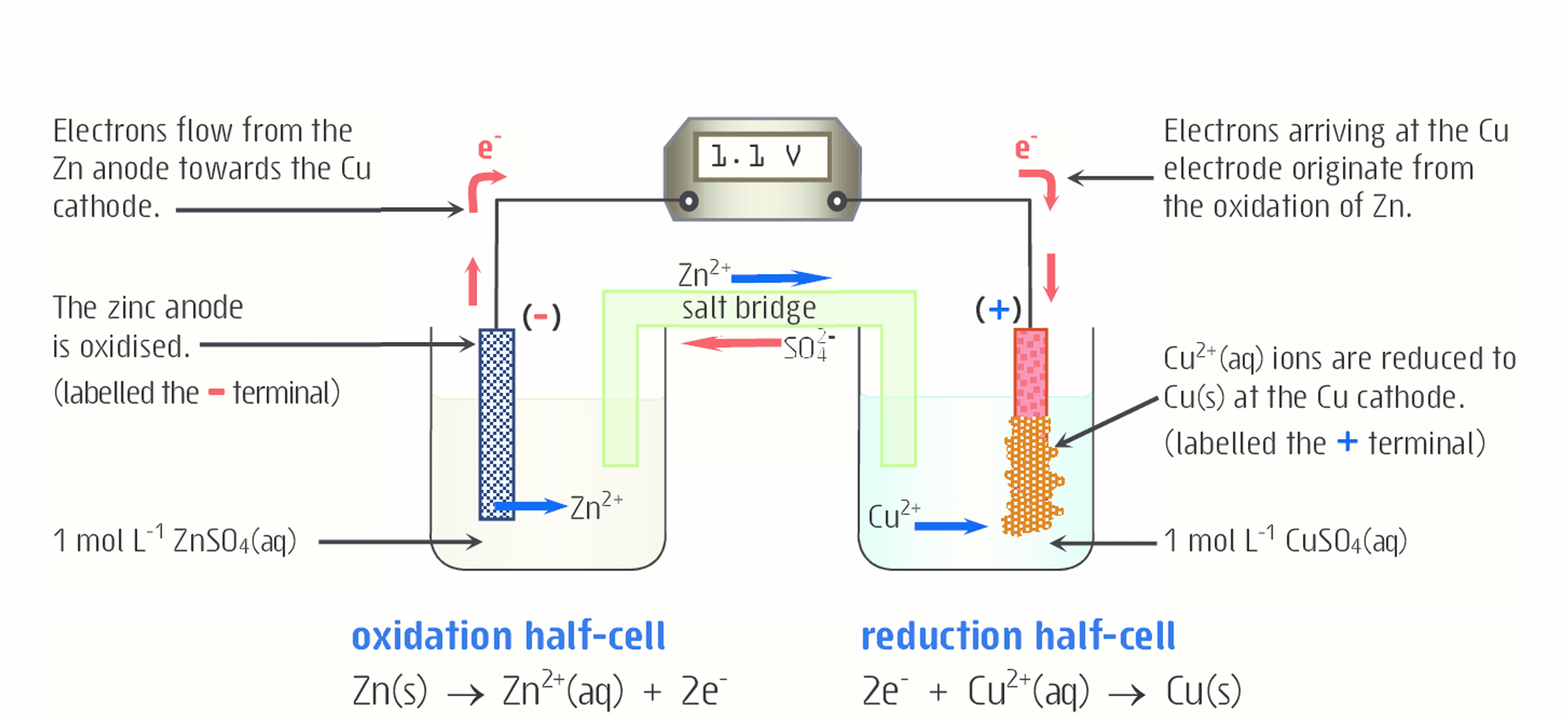
Write the net ionic equation and standard cell potential (E°cell) for that reaction
Zn(s) + Cu2+ (aq) --> Zn2+ (aq) + Cu(s)
The substance with a higher standard reduction potential is reduce so the reaction with a lower reduction potential is reversed
Zn2+ is -0.76 V (without it being reversed), Cu2+ is 0.34 V
E°cell = E°red(cathode) + E°red(anode)
0.34 V + 0.76 V = 1.10 V
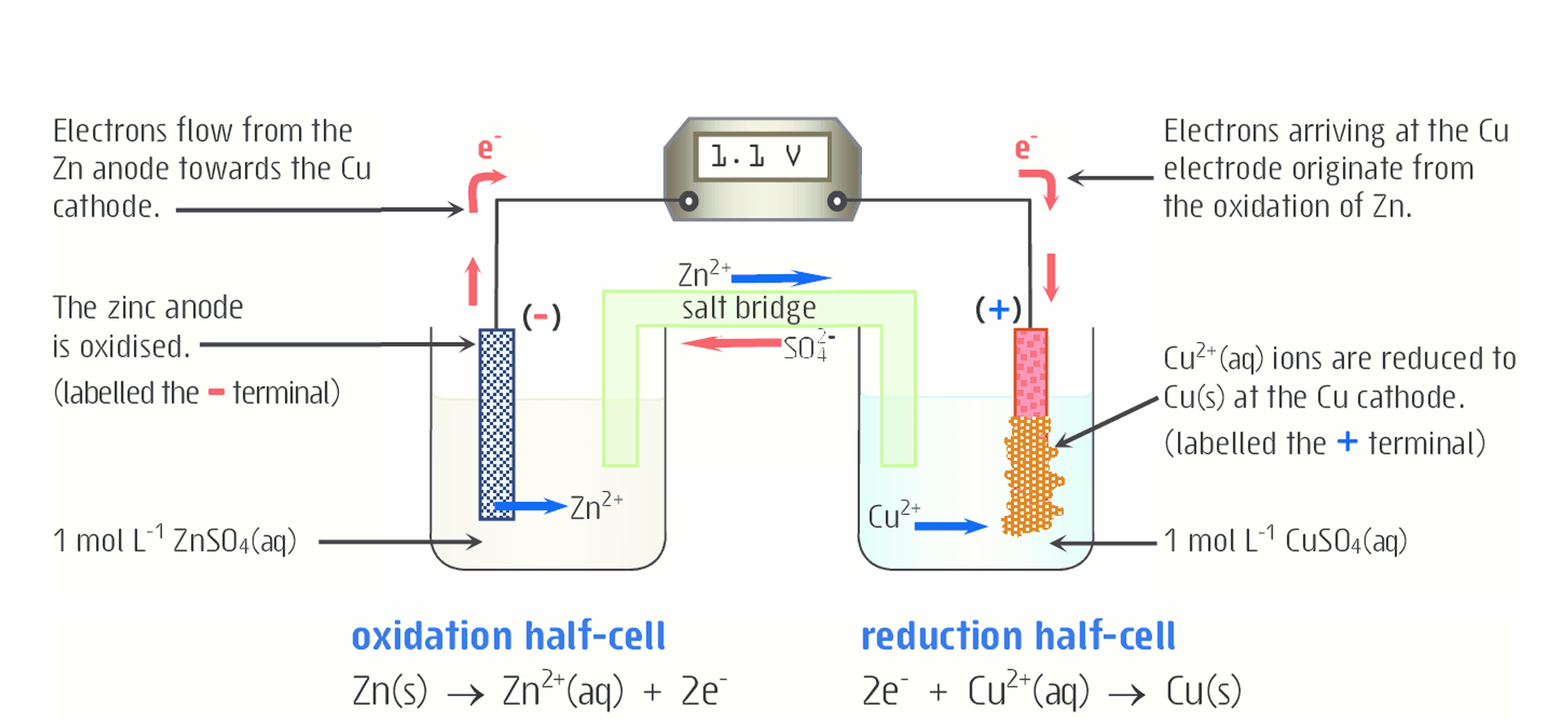
How does the salt bridge work here (for zinc)?
Zn2+ is being produced. This Zn2+ moves into the ZnSO4 solution (which consists of Zn2+ and SO42-). As a result, if not for the salt bridge, the solution would become very positive due to the addition of positively charged ions, so the redox reaction would not occur.
To keep the solution electrically neutral, SO42- ions (anions) move from the salt bridge into the solution (anode). The addition of negative SO42- ions and the addition of positive Zn2+ ions keep the charge from changing significantly and keep the solution neutral.
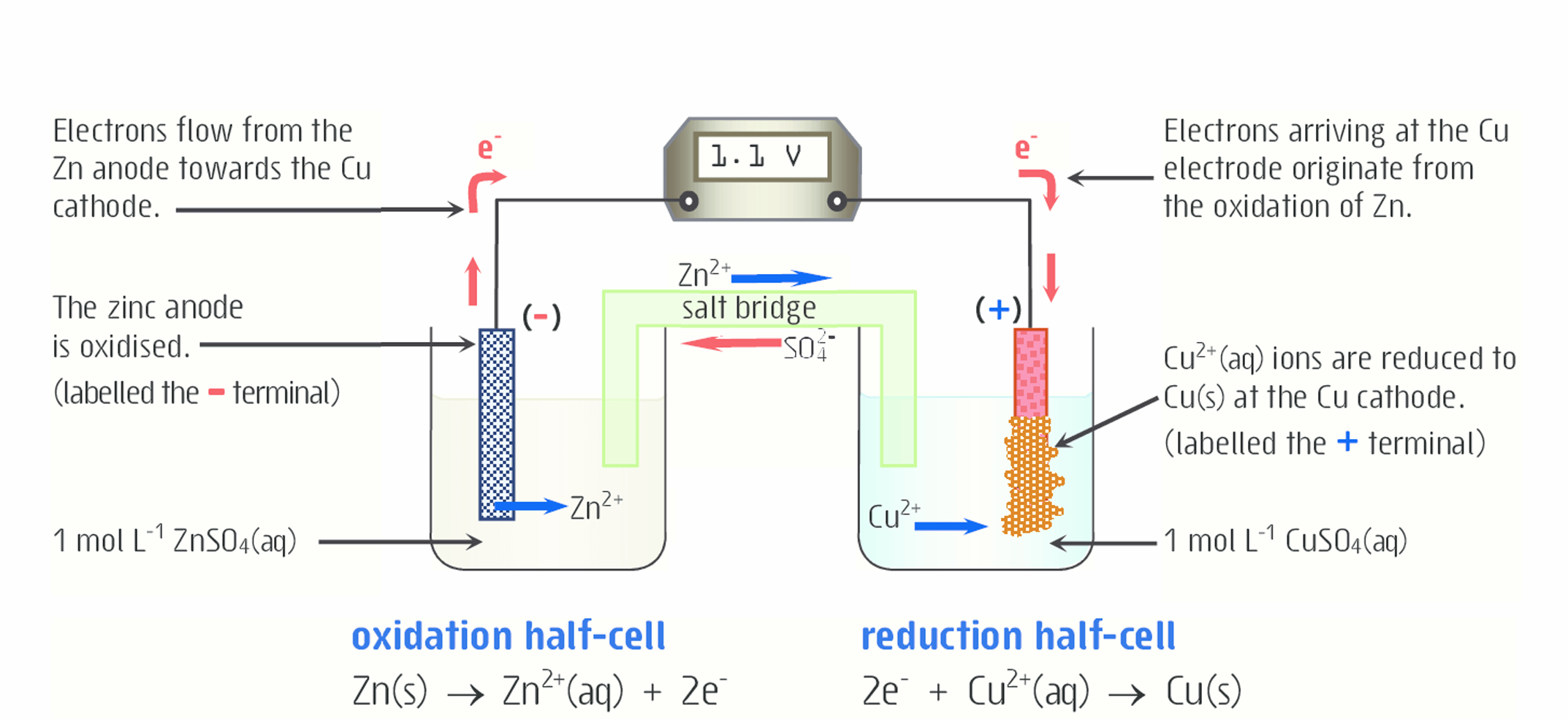
How does salt bridge work here (for copper)?
Cu2+ ions will be removed from the solution and added to the cathode. As a result, if not for the salt bridge, the CuSO4 solution would be very negative because there would be more negative SO42- ions than positive Cu2+ ions, so the redox reaction would not occur. To make up for the loss of positive Cu2+ ions, positive Na+ or K+ ions (cations) ions move from the salt bridge into the solution (cathode)
What is primary cells?
Primary cells are single-use batteries as they cannot be recharged. It contains a fixed amount of redox reactions to produce electricity.
What is an example of a primary cell?
Dry cell produces a maximum voltage 1.5 V, but this voltage produced by the cell decreases over the life of the cell (use in flashlights, portable radios and calculators where low currents are required).
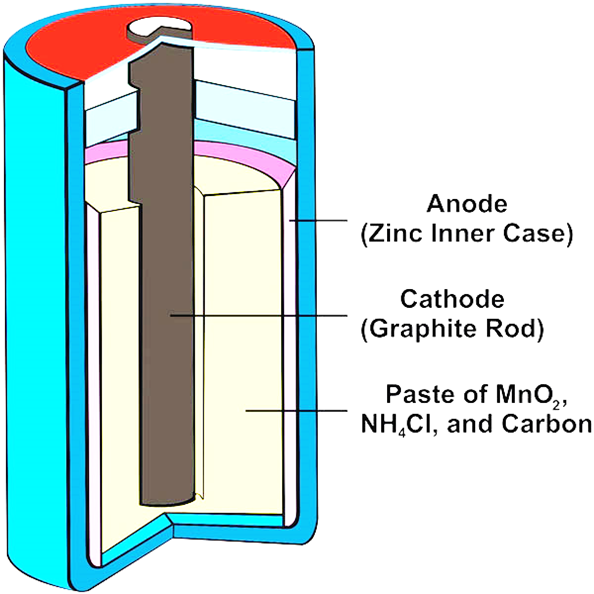
What is the structure of a dry cell?
•The zinc (reducing agent) coating serves as a container (inner case) and the anode.
•The cathode is a graphite rod made of carbon (conducts electricity to the surface of MnO2 particles within the cathode) surrounded by a paste of MnO2 (oxidizing agent)
•NH4Cl (serves as salt bridge needed in the reduction of MnO2) carbon powder, and a small amount of water.
-The ammonium ions are problematic as over time, their acidic nature causes the zinc anode to dissolve into forming Zn2+ ions and result to dry cells limited life span
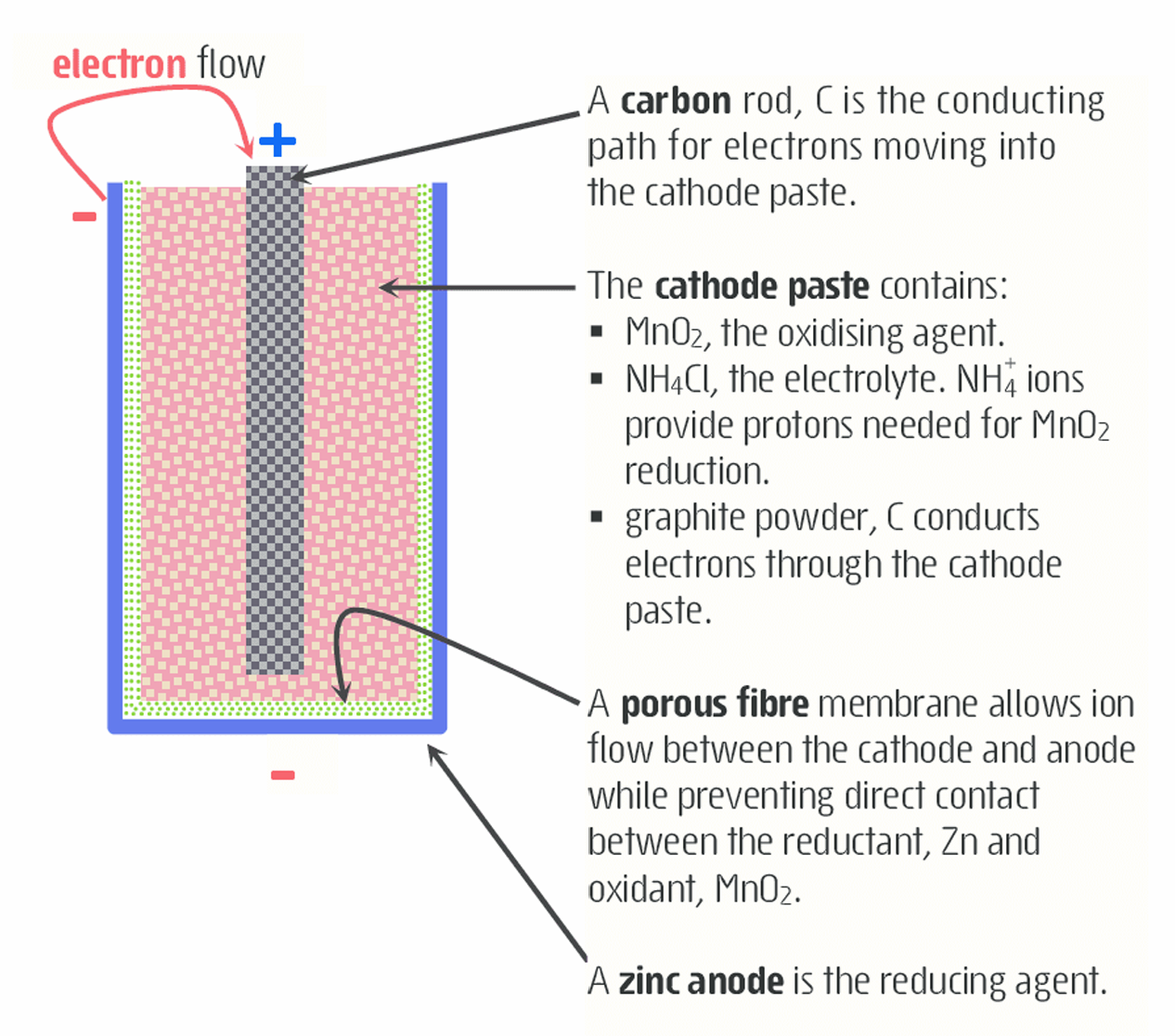
What is the half equation for a dry cell?
(ANODE): Zn(s)⟶Zn2+(aq)+2e−
(CATHODE): 2MnO2(s) + 2NH4(aq) +2e− ⟶ Mn2O3(s) + 2NH3(aq) + H2O(l)
What is the overall equation for a half-cell?
Zn(s) + 2MnO2(s) + 2NH4+(aq) —> Zn2+(aq) + Mn2O3(s) + 2NH3(aq) + H2O(l)
What is another type of dry cell?
Another type of dry cell is the alkaline battery. They produce 3-5 times the energy of a dry cell.
What is the alkaline battery?
Similarly ses Zinc as the reducing agent and MnO2 as the oxidizing agent
Instead of using an ammonium chloride electrolyte, it uses potassium hydroxide in the electrolyte plate
-This eliminates the effect of acidic ammonium ions dissolving the zinc anode of the dry cell
Features allow alkaline cell to achieve a faster reaction rates and give the ability to sustain a high current flow without fall in voltage, which happens in dry cell.
Why do cells alkaline cells have a higher energy density and longer operating life?
Cells have a greater mass of the reductant, Zn and oxidant, MnO2, than a dry cell of similar size.
What is the half equation for alkaline cells?
(ANODE): Zn(s) + 2OH−(aq) ⟶ ZnO(s) + H2O(l) + 2e−
Hydroxide ions because the electrolyte is potassium hydroxide, which is not acidic
(CATHODE): 2MnO2(s) + H2O(l) + 2e− ⟶ Mn2O3(s) + 2OH−(aq)
What is the overall redox equation for a dry cell?
Zn(s) + 2MnO2(s) —> ZnO(s) + Mn2O3(s)
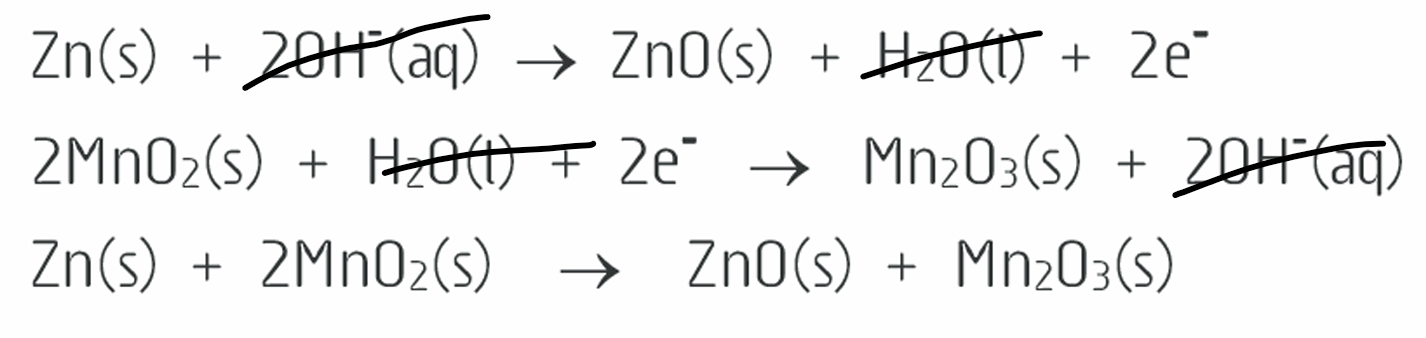
What are alkaline cells used for?
Devices that require a higher current flow, like toys, portable radios, CD players, electronic games and torches.
Alkaline cells are considered non-hazardous waste, and can be hopefully used to be recycled in the future (when a process to recycle them is developed).
What are secondary cells?
The batteries are rechargeable and the cell uses a voltage of 2. Since it is recharge it means that if an electric charge is applied to the cell, it will regenerate the reactants.
Used in the form of a battery consisting of several cells connected in a series (e.g. a car battery uses six of these cell to produce an volatge of 12).
What is an example of an secondary cell?
Lead-car battery (cell) which delivers a very large current. The powdered nature of Pb and PbO2 increases the surface are a and therefore the reaction rate.
-Makes it ideal for running electric starter motor cars and trucks
What does a single lead-acid cell consist of?
Two lead grid electrodes, immersed in an electrolyte of H2SO4(aq)
Anode contains spongy lead while cathode is is packed with lead dioxide.
During discharge, spongy lead becomes oxidized to Pb2+ and then precipitates onto the electrode as insoluble PbSO4(s)
At the same time on the cathode, PbO2+ is reduced to Pb2+, which precipitates onto the electrode as insoluble PBsO4
What is the half equation and full equation for single lead-acid cells?
(ANODE): Pb(s) + SO42-(aq) ⟶ PbSO4(aq) + 2e-
(CATHODE): PbO2(s) + SO42-(aq) + 4H+(aq) + 2e− ⟶ PbSO4(aq) + 2H2O(l)
Full equation: Pb(s) + PbO2(s) + 2SO42-(aq) + 4H+(aq) —> 2PbSO4(aq) + 2H2O(l)
What are fuel cells?
Fuel cells are Galvanic cells which unlike primary and secondary cells, they do not store an oxidising or reducing agent, instead these reactants suppiled to the cell to generate electricity
The chemical by-products of the cell reactions are expelled as the cell operates
What is an example of a fuel cell?
The alkaline hydrogen-oxygen fuel cell
•H2 and O2 are constantly supplied to the cell (gaseous hydrogen as reductant and gaseous oxygen as oxidant)
•The OH- electrolyte interacts with the reactants and H2O is released.
They circulate under pressure over porous nickel electrodes
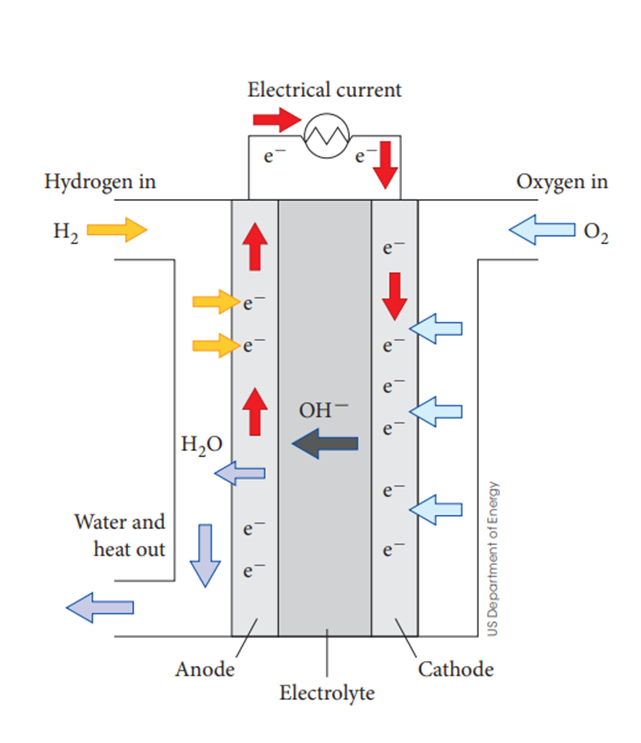
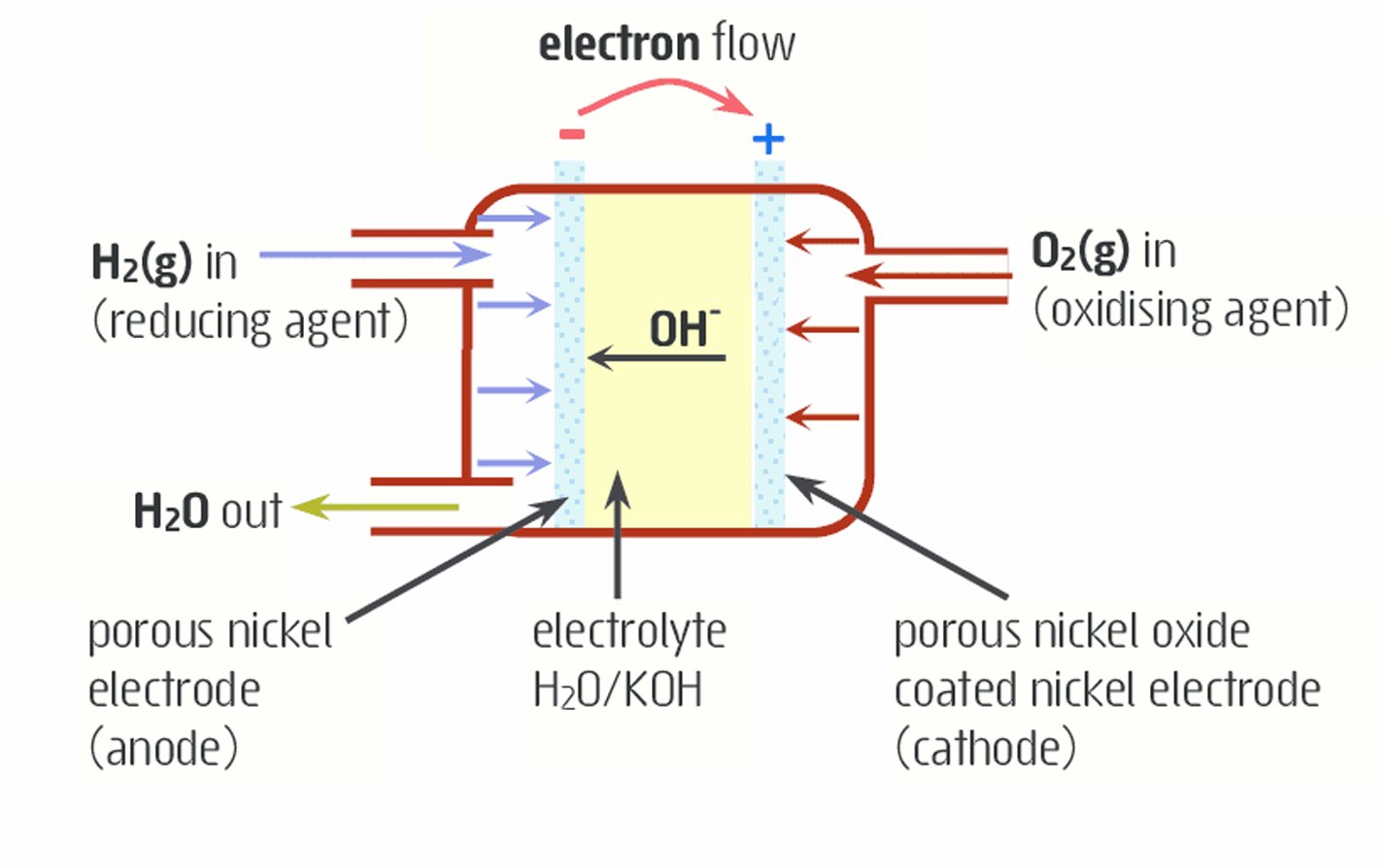
How do alkaline hydrogen-oxygen fuel cells work?
Hydrogen enters the anode and reacts with hydroxide ions from the electrolyte to produce water
This reaction results in the release of electrons
These electrons travel out of the anode, through an external circuit
to create a flow of electricity
These electrons then reach the cathode
Oxygen gas is supplied to cathode, where it combines with water, and accepts electrons to form hydroxide
These hydroxide ions are similarly recycled back to the anode
Water and excess oxygen gas is removed from the cell.
What is the equation for fuel cells?
(ANODE): H2(g) + 2OH-(aq) ⟶ 2H2O(l) + 2e-
(CATHODE): O2(g) + 2H2O(l) + 4e− ⟶ 4OH-(aq)
(OVERALL): 2H2(g) + O2(g) —> 2H2O(l)
What is a hydrogen fuel cell which uses an acidic electrolyte?
H3PO4 is used
H2 and O2 are still continually added
H+ ions interact with reactants to form water

How does an acidic fuel cell work?
A fuel cell works by bringing hydrogen through the anode and oxygen through the cathode. On the anode side, hydrogen molecules are split into electrons and protons. Protons cross the electrolyte membrane, to the cathode (which is negatively charged), while electrons are grouped through a circuit, generating an electric current and excess heat.
On the cathode side, protons, electrons and oxygen combine to produce water molecules.
What is the half and full equation when acidic electrolyte is used?
ANODE): H2(g) ⟶ 2H+(aq) + 2e-
(CATHODE): O2(g) + 4H+(aq) + 4e− ⟶ 2H2O(l)
What is the process of electrolysis?
Carried out by electrolytic cell which consist of a pair of electrodes connected to a electric power supply.
When these electrodes are dipped into a molten or dissolved electrolyte, the energy of the applied DC electric potential causes an non-spontaneous reaction to occur.
What’s the difference between the anode and cathode in an electrolytic cell and a galvanic cell?
Doesn’t use a voltmeter and is replaced by a power supply to provide electricity to the cell
•The anode is still the site of oxidation but in this case, it is the positive electrode. Conducts electrons out of the cell towards the positive terminal of power supply
•The cathode is the site of reduction but is the negative electrode. Conducts electron from the power supply into electrolyte
Does not require a salt bridge
Where do electrons flow in an electrolytic cell?
From the anode to the positive terminal of the power source.
They then flow from the negative terminal of the power source to the cathode.
What happens in the electrolysis using a molten cell?
The innert electrodes (graphite, platinum) are immersed into a vessel of molten salt (inert electrodes do not react).
The power supply is connected causes the electrodes to be oppositely charged and allows the reaction to begin.
•The negative ions in the molten salt migrate to the positive anode and oxidation occurs.
•The positive ions migrate from the salt to the negative cathode and reduction occurs..
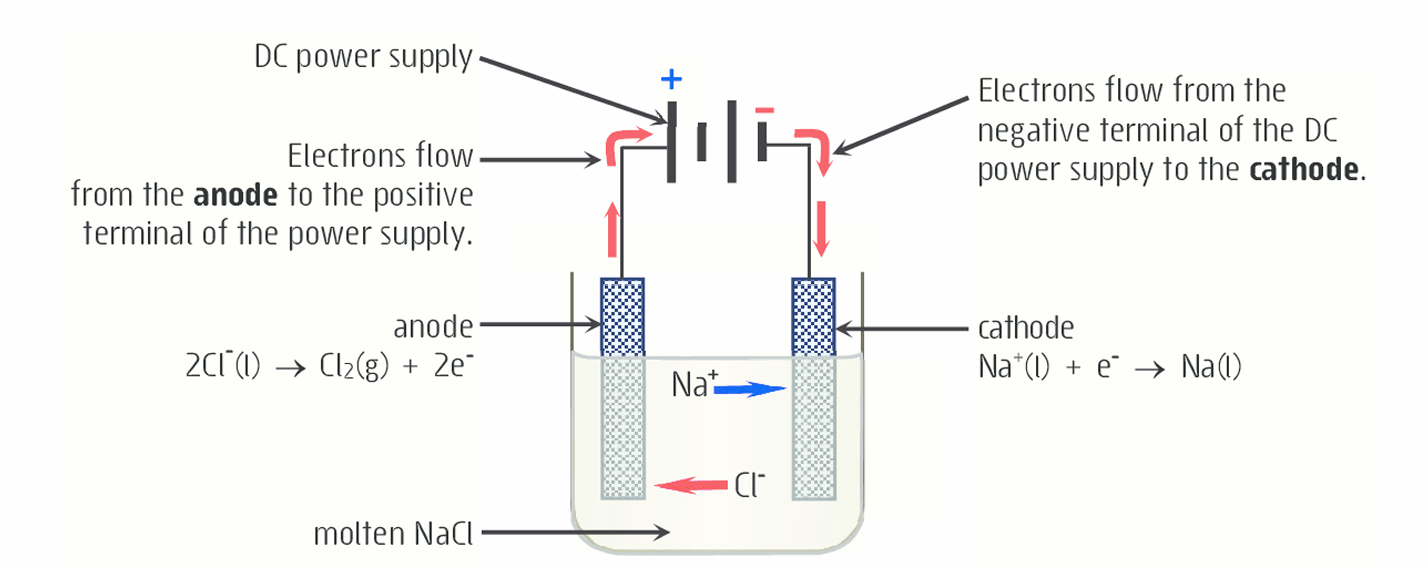
Explain the electrolysis of molten NaCl using inert platinum.
2NaCl(l) —> 2Na(l) + Cl2(g)
Positive sodium ions (Na+) in the electrolyte migrate towards the negative electrode, where they gain an electron to form Na(l).
Negative ions (Cl-) move to the positive electrode, electrode to lose an electron and form Cl2(g)
The DC voltage provides the energy needed to cause the movement of charge
What is the electrolysis of an aqueous electrolyte?
The water from the salt is dissolved it can be preferentially oxidised or reduced.
In a situation where inert electrodes are not used, there is also the potential that the anode itself could be oxidised.
What happens if water is oxidised in an electrolytic cell with an aqueous solution?
When water is oxidized a colorless gas is formed at the anode, and the solution pH falls in the vincity of the anode. The anode reaction is:
2H2O(l) —> O2(g) + 4H+(aq) + 4e- E0 = -1.23 V
If a metallic electrode is oxidised then the anode dissolves and lose mass.
What happens if water is reduced in an electrolytic cell with an aqueous solution?
When water is reduced then a colourless gas is formed at the cathode and pH rises in the vicnity of the cathode. The cathode reaction is:
2H2O(l) + 2e- —> H2(g) + 2OH-(aq) E0 = -0.83 V
If a student performed an experiment of an copper (ll) sulfate solution using using inert platinum electrodes, and then repeated with a pair of copper electrodes, how would the reaction differ?
Plantinum electrodes:
The copper is positive, meaning that it will go to the negative side so the cathode. Since the copper reduction reaction produces an reduction poteintial of +0.34, since it is more positive then the water reduction (which is -0.83), the copper reduction equation is chosen at the cathode.
However, the anode water reaction forming oxygen is chosen.
Both copper electrodes:
Using copper electrodes produced the same observation at the cathode (both reactions were copper). Copper has a more negative reductcion potential of +0.34 compaed to water of +1.23 V, so its chosen at the anode
What does the standard cell potential E0cell tell?
The more positive the standard reduction potential of a substance is, the greater the tendency for the susbtanced to be reduced (greater ability to oxidiser other substances).
A positive value of the E0cell means the redox reaction will be spontaneous while a negative value means the redox reaction will not occur.
-This does not guarantee that a reaction will take place however as if a reaction has a very high activation energy it will not take place even if it has a high E0cell.
Where are the strongest and weakest oxidants found?
•The strongest oxidants can be found in the top left-hand corner of the table.
•The weakest oxidants can be found in the bottom left-hand corner of the table.
Where are the weakest and strongest reductants found?
•The strongest reductants can be found in the bottom right-hand corner of the table.
•The weakest reductants can be found in the top right-hand corner of the table.
What is corrison?
•Corrosion is the surface degradation of metallic substances caused by their oxidation in the presence of oxygen (in air).
•Corrosion reactions are spontaneous redox reactions but take a long time to react.
•Water vapour in the air acts as the electrolyte through which charged particles (electrons) are carried.
What is the most common form of corrison and the equation?
The most common form of corrosion is the corrosion of iron-based materials to form rust. The reddish rust that occurs on metals is hydrated iron (III) oxide.
What is the half and over all equation for iron-based materials?
At the anodic sites, iron loses electrons and undergoes oxidation to produce ferrous ions and releases electrons:
Fe(s) → Fe2+(aq) + 2e-
The electrons released at the anode travel through the iron to the cathodic sites, where they reduce oxygen in the presence of water:
O2(g) + 2H2O(l) + 4e- → 4OH-(aq)
Full equation:
2Fe(s)+4H+(aq)+O2(g)→2Fe2+(aq)+2H2O
iron can precipitate:
4Fe(s)+O2(g)+7 H2O(l)→2Fe2O3⋅32H2O(s)+8H+(aq)
Simplified:
4Fe(s) + 3O2(g) —> 2Fe2O3(s)
How does rust form?
The iron (Fe2+) produced at the anode react with the hydroxide ions (OH−) produced at the cathode to form iron(II) hydroxide:
Fe2+ (aq) + 2OH−(aq) → Fe(OH)2(aq)
Iron(II) hydroxide (Fe(OH)2) is unstable and further reacts with oxygen and water to form iron(III) hydroxide (Fe(OH)3):
4Fe(OH)2(aq) + O2(g) + 2H2O(l) → 4Fe(OH)3(aq)
Iron(III) hydroxide (Fe(OH)3) dehydrates to form hydrated iron(III) oxide, commonly known as rust:
Fe(OH)3(aq) →Fe2O3⋅nH2O(s)
What are factrs that accelerate corrison?
1.Presence of Water: Water is essential for rusting as it facilitates the electrochemical reactions involved.
2.Oxygen: Increased exposure to air, especially in environments with high oxygen levels, accelerates rust formation.
3.Salt: Salt, particularly sodium chloride, accelerates rusting by increasing the conductivity of water, which enhances the electrochemical reactions.
4.Acids: Acidic environments, such as those with acid rain or industrial pollutants, can increase the rate of rusting. Acids provide hydrogen ions that facilitate the oxidation of iron.
5.Temperature: Higher temperatures generally increase the rate of chemical reactions, including rusting.
What is the barrier method for ion to control corrrison?
A barrier method is on where a coating is applied to the iron to prevent oxygen and water from contacting the iron.
Adding paint, oil/grease or an inert coating such as tin can act as a physical barrier between the metal and corrosive agents such as water, oxygen, and salts. This prevents the electrochemical reactions that lead to corrosion.
-Tin has a higher reduction potential meaning that iron is oxidised.
Prevents moisture and oxygen from reacting with the metal.
What happens if the coating is damaged in sacrifical method>
If the coating is damaged and the iron is exposed:
•Oxygen and water will oxidise the iron, causing it to corrode.
•The tin will remove electrons from the iron and will cause it to be corroded at a higher rate.
What is the sacrifical method of controlling corrision?
•Sacrificial methods of controlling corrosion involve using a more reactive metal to protect a less reactive metal from corroding (two metals are touching or connected electrically)
•The sacrificial anode oxidises, supplying electrons to the protected metal.
•Oxygen and water use these surplus electrons to undergo reduction (instead of iron breaking down to provide them).
•The sacrificial anodes need to be replaced over time to ensure there is sufficient material to be oxidised.
•Common sacrificial anodes include zinc, magnesium, and aluminium.
What is cathodic protection?
•The use of a sacrificial anode is known as cathodic protection, because it forces the iron to become the cathode, protecting it from corrosion.
•Cathodic protection involves the supply of electrons to the iron to prevent them from losing the electrons
What is another method of cathodic protection?
•Impressed current cathodic protection, where an external voltage supply is connected to the iron, providing it with electrons and thus protecting it from corrosion.
•The negative terminal is connected to the protected iron and the positive terminal is connected to a piece of scrap iron, which will be preferentially oxidised.
What is Galvanizing (using both barrier and sacrificial methods)?
This involves coating the iron with a more reactive metal such as zinc.
•This limits the interaction of oxygen and water with the surface (barrier method).
•If the surface is damaged, and the iron is exposed, the zinc is more likely to be oxidised (sacrificial method).
The zinc doesn’t oxidise because it forms a layer of ZnCO3.
Zn(OH)2 on the surface which protects it from corrosion
What are sliver oxide button cells?
Small primary cells with a constant voltage of 1.86 V
Used in cameras, watches and hearing age
Greater energy density than the alkaline cell
What is the chemical reaction for sliver oxide cells?
Anode: Zn(s) + 2OH-(aq) —> ZnO(s) + H2O(l) + 2e-
Cathode: Ag2O(s) + H2O(l) + 2e- —> 2Ag(s) + 2OH-(aq)
Overall redox reaction: Zn(s) + Ag2O(s) —> ZnO(s) + 2Ag(s)
What are lithium cells?
The high oxidation potential and low density of lithium makes it ideal to be used as the reducing agent for galvanic cell
Lithium anode and manganese dioxides at the cathode (oxidizing agent)
What is lithium cell half and overall equation?
Anode: Li(s) —> Li+(l) + e-
Cathode: MnO2(s) + Li+(l) + e- —> LiMnO2(s)
Overall: Li(s) + MnO2(s) —> LiMnO2(s)
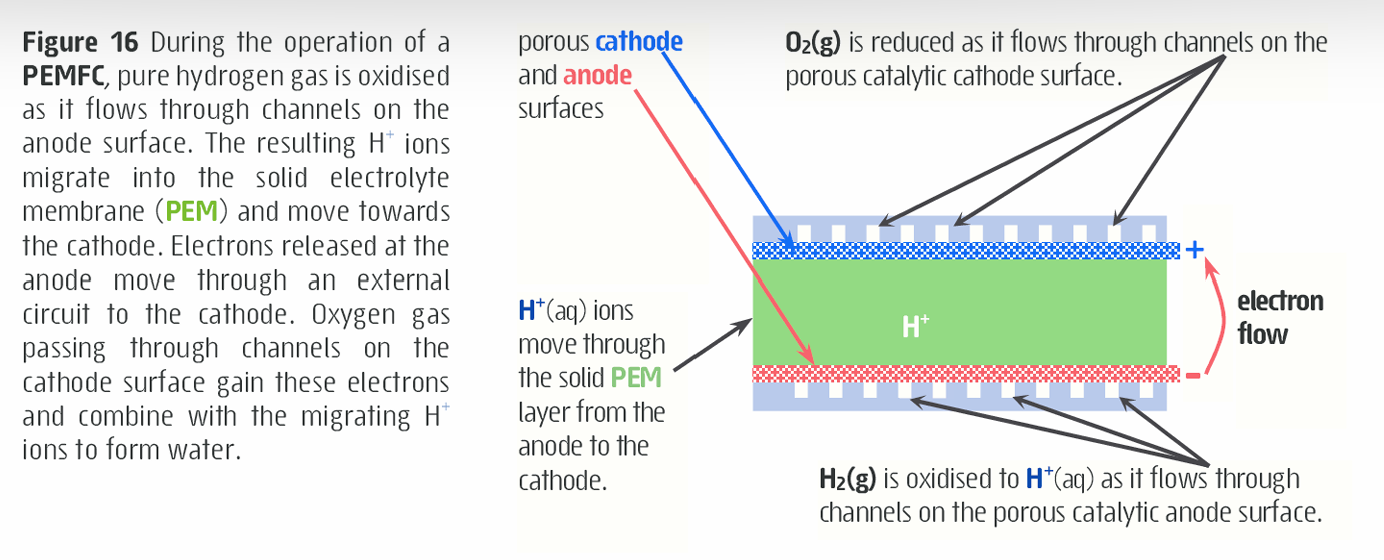
What is proton exhcnage membrane fuel cell?
Uses 99.999% pure H2(g) and O2(g) from the air to produce a DC current, water and heat.
Solid polymer protein exchange membrane is used as the electrolyte and electrode separate (avoid the use of corrosive liquid electrolyte)
The solid protein exchange membrane allows protons (H+ ions) to move through it from the anode to the cathode while preventing H2(g) and O2(g) from moving across the thin, solid electrolyte membrane
Platinum acts as a catalyst to speed up the anode and cathode reaction
How does a proton exchange membrane fuel cell work?
Pure hydrogen gas is oxidized as it flows through channels on the anode surface
Results in H+ ions to migrate into the solid electrolyte membrane (PEM) and move towards the cathode
Electrons released at anode move through an external circuit to the cathode
Oxygen gas passing through channels on the cathode surface gain these electrons and combine with the migrating H+ ions to form water.
What is the equation for a proton exchange membrane fuel cell?
Anode half-reaction: H2(g) —> 2H+(aq) + 2e-
Cathode half-reaction: O2(g) + 4H+(aq) + 4e- —> 2H2O(l)
Overall: 2H2(g) + O2(g) —> 2H2O(l)
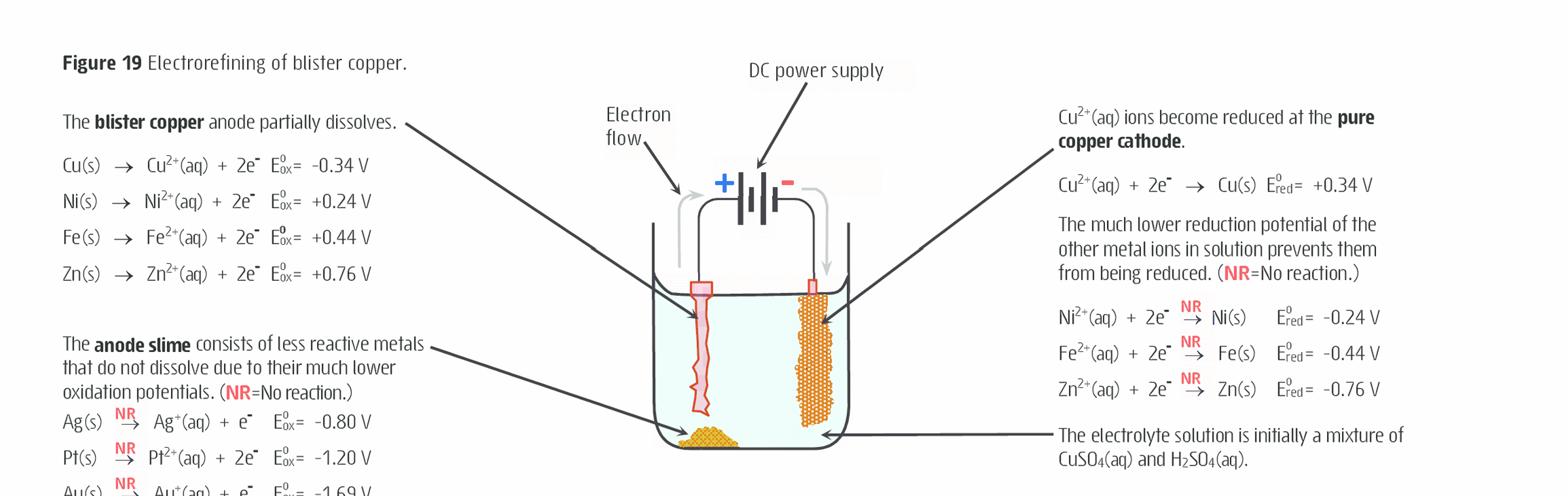
What is the electroefining of copper?
Copper high resistance to corrision but small impurities can diminish these attributes
Impure form of copper is called blister copper and impurities include metals like Ag, Au, Fe, Zn and Ni.
Purification of blister copper to achieve >99.95% is achieved by electrofining (electrolysis)
Blister coppper froms the anode and immersed into an electrolyte solution of copper (II) sulfate dissolved in the dilute sulfuric aid and loses an electron
DC voltage to the impure anode results in the oxidation (dissolving) of copper and the more reactive metal impurities
Cu²⁺ ions in the solution gain electrons and stick to the cathode (reduction) which is made up of pure copper
This slowly builds up a layer of pure copper.
The less reactive metals fall to the bottom asnd form a solid known as anode slime
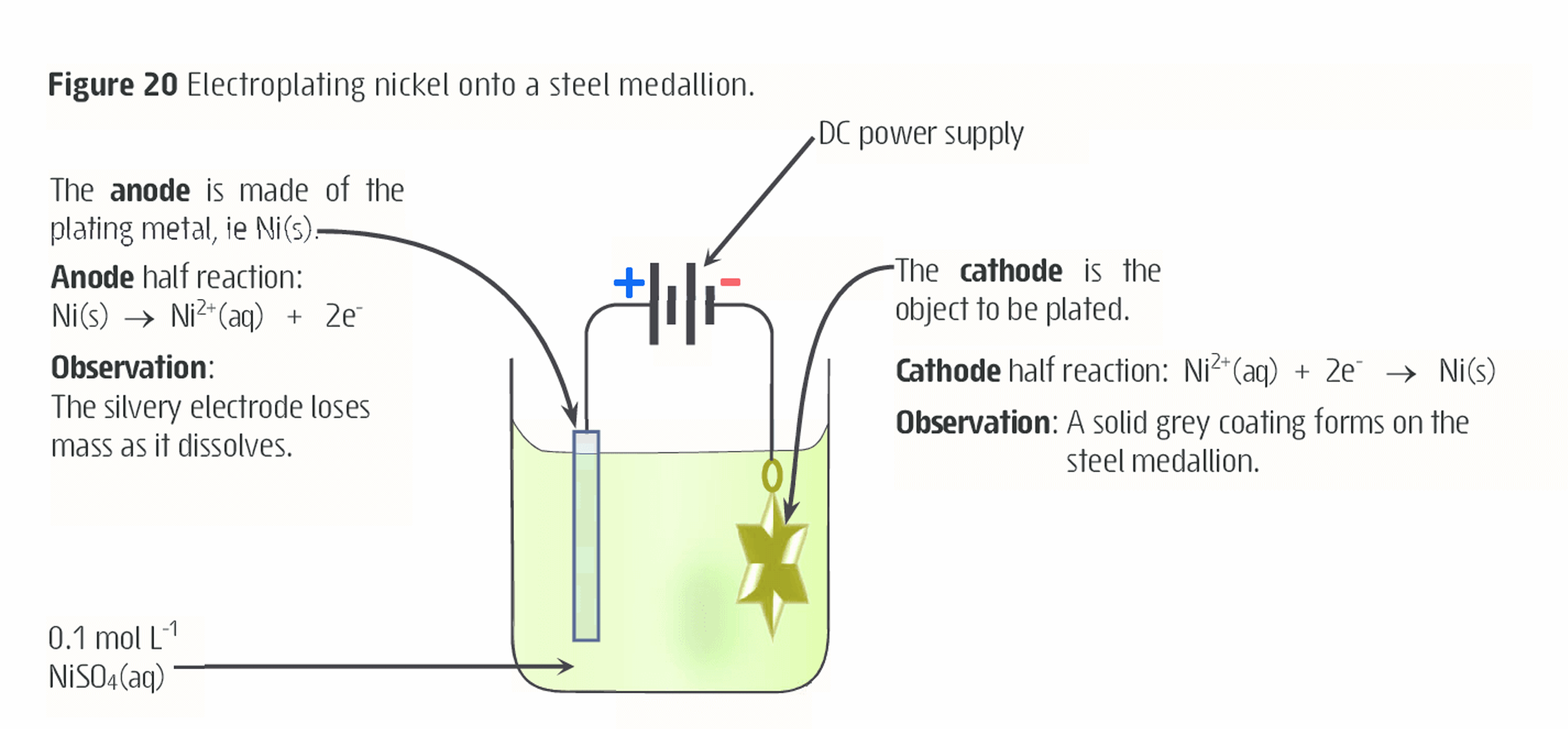
What is electroplating?
Using electrolysis to place a thin coating of one metal onto another metal
Achieved by making the metal object to be plated the cathode of an electrolytic cell
The anode is made of the plating metal while the electrolyte is a salt of the same metal
DC voltage causes to the electrodes causes the metal ions from the electrolyte to become reduced on the cathode
As the cell operates, oxidation of the anode replaces the reduce metal ions ensuring a steady concentration of these is maintained within the electrolyte
(WHEN ELECTRONS ARE GAINED AT THE CATHODE THE METAL IONS IN ELECTROLYTE MOVE TO THE CATHODE CAUSING IT TO BE COATED)
What is electroplating used for?
Improved Appearance:
Electroplating can add a decorative finish to objects, making them look more appealing.
Increased Durability:
The coating can protect the underlying material from wear and tear, increasing its lifespan.
Corrosion Resistance:
Electroplating can help prevent rust and other types of corrosion, especially when metals like chromium, zinc, or copper are used as coatings.
Enhanced Functionality:
In some cases, electroplating can improve the electrical conductivity, friction, or other functional properties of the object.
Cost-Effectiveness:
By using a less expensive base material and then electroplating it with a more durable or attractive metal, manufacturers can achieve desired properties without incurring the high costs of using the more expensive metal throughout the entire object.